Introduction
Behçet’s disease (BD) is a long-term, multisystemic illness characterized by a variety of symptoms, including relapsing-remitting oral ulcers, genital ulceration, ocular involvement, skin lesions, and numerous manifestations [1]. Behçet’s disease is more common among nations across the old Silk Route that extended from Eastern Asia to the Mediterranean area [2]. In a large multicenter study in BD, the male-to-female ratio was estimated to be 2.6 to 1, and there was a gender-driven effect on illness phenotype [3]. Ocular involvement is the presenting feature in fewer than 20% of all BD diagnoses, while 70% of patients report eye symptoms around 2–4 years from the onset of the disease [4], with a prevalence that ranges from 40 to 70% [5, 6].
Ocular involvement compromises bilateral recurring non-granulomatous pan-uveitis as well as occlusive retinal vasculitis. Bouts of inflammation that affect the posterior segment can cause irreversible visual impairment owing to involvement of the retina that is accompanied by macular scarring, together with optic atrophy [7]. Homocysteine [8] and apoptosis markers [9] were identified to take part in ocular involvement and uveitis in BD patients.
A proliferation-inducing ligand (APRIL), also referred to as tumor necrosis factor ligand superfamily member thirteen (TNFSF13), is a tumor necrosis factor (TNF) superfamily protein which binds to cell surface receptors. The APRIL gene is found on human chromosome 17 [10]. It is regarded as a critical survival factor during B-cell maturation and plays a vital role in the formation of these cells tolerance. It affects the content and size of the B-cell compartment that is a key driver of hyperplasia and auto-antibody synthesis by B-cells in auto-immune disorders [11].
Serum APRIL concentrations have been reported to be elevated in individuals diagnosed with the auto-immune disorders systemic lupus erythematosus [12], IgG4 related-diseases [13], and primary Sjögren’s syndrome [14]. However, it is currently unclear whether APRIL has a role in auto-immunity in people with BD, and whether its circulating level correlates with ocular involvement.
The objective of the study was to find out whether serum concentrations of APRIL are associated with ocular involvement in BD patients.
Material and methods
Study population
Sixty patients who fulfilled the diagnostic International Criteria for Behçet’s Disease (ICBD) [15], were recruited from Rheumatology and Ophthalmology out-patient clinics, Minia University Hospital, from January 2023 to November 2023. Thirty age- and sex-matched healthy individuals were enrolled as a control group. Patients were excluded if they were under the age of 18, had other connective tissue disease or vasculitis, had diabetes or hypertension, had malignancy, had a past history of intraocular surgery, or had precluding problems such as media opacity and severe glaucoma.
Clinical assessments
Complete history taking, thorough clinical examination, patients’ medications were documented, and disease activity was evaluated using the Behçet Disease Current Activity Form (BDCAF) [16]. A rheumatologist performed a clinical examination on each patient, followed by an ophthalmologist’s evaluation.
Ophthalmological assessment
All patients underwent thorough ophthalmological examination including unaided visual acuity, best corrected visual acuity, refraction utilizing an autorefractometer, full slit-lamp biomicroscopy, intraocular pressure applying Goldmann applanation, ocular ultrasonography, and optical coherent tomography.
Investigations
Laboratory tests including complete blood count, first hour erythrocyte sedimentation rate (ESR) (Westergren), C-reactive protein (CRP), serum aspartate transaminase and alanine transaminase, blood urea and serum creatinine were performed for all patients. Both patients and controls had their serum APRIL levels measured using the ELISA method.
Statistical analysis
Analysis of the study data was done with IBM SPSS Statistics version 20 for Windows. Categorical data are presented as numbers and percentages. The χ2 test compared two sets of categorical data. The quantitative data were tested for a normal distribution using the Kolmogorov-Smirnov test. For quantitative variables that followed a normal distribution the three groups were compared using the one-way ANOVA test. Numerical data were correlated using either Pearson’s or Spearman’s correlation (r). The ultimate cut-off level of serum APRIL that can differentiate patients from controls, and differentiate patients with ocular involvement from those without, was determined using receiver operating curve (ROC) analysis. The dependent and independent risk variables were examined using logistic regression analysis. The p-value is always 2-tailed and significance was set at the < 0.05 level.
Results
Among the 60 BD studied patients, there were 36 men (60%) and 24 women (40%) with a ratio 1.5 : 1. The mean age of the patients was 37.1 ±5.59 years, ranging from 28 to 52 years, vs. 35.23 ±5.05 years in the controls, and the mean disease duration was 5.8 ±3.99 years, ranging from 1 to 17 years, with a mean age at onset of 31.3 ±6.1 years, ranging from 19 to 44 years.
When the study was conducted, 34 patients (56.7%) were receiving steroids, 32 (53.3%) were on immunosuppressive drugs (azathioprine or cyclophosphamide), 30 patients (50%) were taking adalimumab, 29 patients (48.3%) were on colchicine and only 9 patients (15%) received cyclosporine.
One or more ocular manifestations were found in 42 BD patients (70%), while 18 patients (30%) had no ocular involvement. There was a no significant difference regarding sex distribution, age of onset, or disease duration between patients with ocular disease and patients without ocular disease. Among the 42 BD patients who had ocular involvement, bilateral eye involvement was reported in 33 patients (78.6%) and unilateral eye involvement in 9 patients (21.4%) with complete visual loss in three eyes out of 84 eyes.
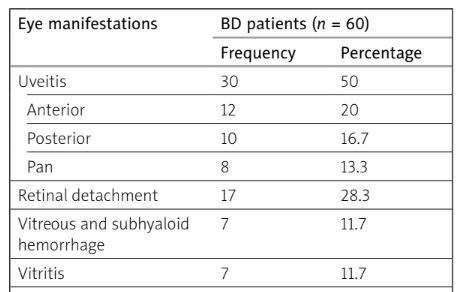
Uveitis was the most prevalent sign in our BD patients, being reported in 50% of the patients, with 12 patients (20%) having anterior uveitis, 10 patients (16.7%) having posterior uveitis and 8 patients (13.3%) developed panuveitis. Retinal detachment was reported in 17 patients (28.3%), and vitreous with subhyaloid hemorrhage and vitritis were found in 7 patients each (11.7%). Corneal ulcers, retinal vasculitis with optic atrophy, and cataract were detected in 6 patients each (10%), lamellar hole in 5 patients (8.3%), while hypopyon, neovascular glaucoma and macular degeneration were documented in 3 patients each (5%) (Table I).
Table I
Frequency of ocular involvement in patients with BD (n = 60)
The mean level of serum APRIL was statistically significantly higher in BD patients, with a mean of 39.48 ±9.29 ng/ml, than in controls, with a mean of 4.83 ±1.67 ng/ml (p < 0.001). By analyzing serum APRIL concentrations in BD patients based on the existence of ocular involvement, we found a statistically significantly higher serum APRIL in BD patients with ocular involvement than those without (43.91 ±7.38 ng/ml vs. 29.16 ±2.67 ng/ml, p < 0.001). For testing the differences in the means of serum APRIL among the three groups, BD patients with ocular involvement, BD patient without ocular involvement, and healthy controls, we used the one-way analysis of variance (ANOVA), and the difference was highly statistically significant (p < 0.001) (Table II).
Table II
Mean values of serum APRIL in the studied groups (n = 90)
| Serum APRIL | One-way ANOVA | ||
|---|---|---|---|
| Mean ±SD | F | p | |
| BD with ocular involvement (n = 42) | 43.91 ±7.38 | 478.39 | 0.0001 |
| BD without ocular involvement (n = 18) | 29.16 ±2.67 | ||
| Healthy controls (n = 30) | 4.83 ±1.67 | ||
Among different clinical manifestations, serum APRIL levels were statistically significantly higher in BD patients with oral ulcers, arthritis, patients who experienced cutaneous involvement, and those who had ocular involvement in general, and uveitis in particular, either anterior, posterior or panuveitis (p < 0.005, p < 0.005, p < 0.05, respectively). In addition, serum APRIL levels were significantly higher in patients with hypopyon (p < 0.05) and cataract (p < 0.05) (Table III).
Table III
Comparison between mean values of serum APRIL level in BD patients with different clinical and ocular manifestations (n = 60)
| Serum APRIL, mean ±SD | Difference | ||
|---|---|---|---|
| t | p | ||
| Oral ulcer | |||
| Absent (n = 3) | 39.48 ±9.50 | 2.734 | 0.023* |
| Present (n = 57) | 45.97 ±9.05 | ||
| Genital ulcer | |||
| Absent (n = 24) | 37.67 ±10.57 | –1.181 | 0.244 |
| Present (n = 36) | 40.69 ±8.27 | ||
| Cutaneous involvement | |||
| Absent (n = 40) | 37.79 ±9.97 | –2.324 | 0.024* |
| Present (n = 20) | 42.87 ±6.79 | ||
| Central nervous system involvement | |||
| Absent (n = 30) | 38.70 ±9.33 | –0.648 | 0.519 |
| Present (n = 30) | 40.27 ±9.34 | ||
| Vascular involvement | |||
| Absent (n = 34) | 38.17 ±9.45 | –1.265 | 0.211 |
| Present (n = 26) | 41.20 ±8.97 | ||
| Arthritis | |||
| Absent (n = 42) | 32.13 ±1.19 | –2.444 | 0.018* |
| Present (n = 18) | 44.95 ±9.01 | ||
| Ocular involvement | |||
| Absent (n = 18) | 29.16 ±2.67 | 11.338 | 0.0001** |
| Present (n = 42) | 43.91 ±7.38 | ||
| Anterior uveitis | |||
| Absent (n = 48) | 38.13 ±9.40 | –2.878 | 0.008** |
| Present (n = 12) | 44.92 ±6.70 | ||
| Posterior uveitis | |||
| Absent (n = 50) | 38.26 ±9.50 | –3.543 | 0.002** |
| Present (n = 10) | 45.59 ±4.97 | ||
| Pan-uveitis | |||
| Absent (n = 52) | 38.62 ±9.31 | –2.253 | 0.046* |
| Present (n = 8) | 45.14 ±7.33 | ||
| Corneal ulcers | |||
| Absent (n = 54) | 38.96 ±9.43 | –1.677 | 0.136 |
| Present (n = 6) | 44.17 ±6.92 | ||
| Hypopyon | |||
| Absent (n = 57) | 38.96 ±9.20 | –3.997 | 0.024* |
| Present (n = 3) | 49.50 ±4.05 | ||
| Retinal vasculitis | |||
| Absent (n = 54) | 38.83 ±9.27 | –1.909 | 0.100 |
| Present (n = 6) | 45.40 ±7.85 | ||
| Vitreous and subhyaloid hemorrhage | |||
| Absent (n = 53) | 39.54 ±9.48 | 0.242 | 0.815 |
| Present (n = 7) | 38.76 ±8.33 | ||
| Retinal detachment | |||
| Absent (n = 43) | 38.25 ±9.92 | –1.964 | 0.056 |
| Present (n = 17) | 42.62 ±6.73 | ||
| Vitritis | |||
| Absent (n = 53) | 39.15 ±9.56 | –1.140 | 0.278 |
| Present (n = 7) | 42.04 ±5.73 | ||
| Optic atrophy | |||
| Absent (n = 54) | 39.33 ±9.71 | –0.732 | 0.478 |
| Present (n = 6) | 40.90 ±4.15 | ||
| Cataract | |||
| Absent (n = 54) | 38.55 ±9.11 | –3.080 | 0.017* |
| Present (n = 6) | 47.88 ±6.77 | ||
| lamellar hole | |||
| Absent (n = 55) | 39.50 ±9.44 | –0.732 | 0.478 |
| Present (n = 5) | 39.30 ±8.39 | ||
| Neovascular glaucoma | |||
| Absent (n = 57) | 39.27 ±9.44 | –0.051 | 0.961 |
| Present (n = 3) | 43.67 ±5.09 | ||
| Macular degeneration | |||
| Absent (n = 57) | 39.22 ±9.38 | –1.371 | 0.281 |
| Present (n = 3) | 44.57 ±6.40 | ||
Although serum APRIL level was higher in BD patients compared to the control group, no correlation was found between APRIL level and BDCAF sore. Additionally, no correlation was found between serum levels of APRIL and glucocorticosteroids or immunosuppressant drug usage. No correlation was found between APRIL level and any of the laboratory parameters except for ESR (r = 0.29, p < 0.05) and CRP (r = 0.31, p < 0.05).
When we applied logistic regression analysis, cutaneous lesions and arthritis were strong independent predictors for ocular involvement in our BD patients. The existence of skin lesions increases the risk of ocular involvement by 47-fold, while the presence of arthritis increases the risk of ocular involvement by 22-fold (Table IV).
Table IV
Logistic regression analysis showing risk factors of ocular involvement in BD patients
A ROC curve was constructed, and serum APRIL at a cut-off point of 11.5 ng/ml could distinguish BD patients from controls. A cut-off point of 33.4 ng/ml could distinguish between BD patients with and without ocular affliction with a sensitivity of 97.6%, specificity of 94.4%, and area under the curve (AUC) of 0.976 (Table V).
Table V
Sensitivity and specificity for serum APRIL in detecting ocular involvement in BD patients
Discussion
Behçet’s disease is a chronic multi-systemic disease featured by relapsing-remitting systemic manifestations, frequent visual system abnormalities, and symptoms of neurological and other internal organs [17]. However, some findings, such as uveitis flares, vascular or neurologic symptoms, may result in irreversible tissue damage, increasing morbidity and mortality [18].
Ocular involvement is prevalent in BD, results in significant functional impairments [19], and is thought to be a major contributor to patient morbidity [20]. In the present study, one or more ocular impairment was reported in 42 of our BD patients (70%), in alignment with the prevalence found in a multicenter nationwide study on 1526 adult patients with BD which reported ocular involvement in 70.8% of their studied population [3], and a prevalence of 73.7% in the El-Najjar study [4]. Our finding was slightly lower than the prevalence of 80% in a Brazilian study of BD patients [21]. How-ever, in 2014, Shaker et al. reported that only 40% of patients with BD had ocular involvement [22]. Likewise, Allam et al. [8] reported a prevalence of 44.4% of patients with BD.
Uveitis was the most common ocular involvement, being noted in 50% of our BD patients, with 20% having anterior uveitis, 16.7% having posterior uveitis, and 13.3% having panuveitis. Gheita et al. [9] in 2013 reported that anterior uveitis was the commonest, being detected in 32.7% of BD patients, followed by posterior uveitis in 22.4%, and 17.2% developed panuveitis, which agrees with our results. Our finding was lower than the prevalence of uveitis of 85% in a study of 40 BD patients [23]. However, a recent study in 2024 found that panuveitis was the most prevalent presentation, accounting for 64.4%, whereas 22.6% had isolated posterior uveitis and 12% had anterior uveitis [24].
As a matter of fact, BD can affect several parts of the eye, with uveitis being the most prevalent eye involvement. Other major problems include retinal detachment, hemorrhage, and optic nerve injury. The variation in the prevalence of ocular involvement might be attributed to the disease’s nature, marked by periods of exacerbation and remission. The frequency and severity of these flares might differ amongst patients, influencing the progression and severity of ocular symptoms over time. In addition, data on the optimal duration of immunosuppressive medication in patients with ocular BD are scarce. As a result, the choice to escalate or deescalate therapy based on the severity of the ocular clinical presentation remains not fully elucidated in the majority of studies.
Among different clinical manifestations of BD, serum APRIL level was statistically significantly raised in BD patients manifesting arthritis; moreover, serum APRIL was positively correlated with arthritis. Our results are congruent with those reported by Koyama et al. [12], who studied serum APRIL in SLE patients and observed a strong correlation between serum APRIL levels and musculoskeletal involvement in SLE, primarily arthritis. It has been observed that TACI-immunoglobulin, an APRIL and BAFF antagonist, can reduce joint inflammation and degeneration in collagen-induced arthritis, a type of noninfectious inflammatory arthritis [25]. Additionally, it had been shown that BLyS and APRIL can control T and B cell activity and may possess pro-inflammatory properties in the presence of synovitis in rheumatoid arthritis patients [26]. In individuals with inflammatory arthritis, APRIL and BAFF levels were found to be higher in the synovial fluid but not in the serum, indicating enhanced local synthesis of that complex in the presence of arthritis [27].
Based on the current study, BD patients with cutaneous involvement had statistically significantly higher serum APRIL levels than those without. Analogous research revealed that individuals suffering from vitiligo and atopic dermatitis had greatly raised levels of serum APRIL [28]. It was proposed that keratinocytes in the skin, in addition to providing a barrier function, may produce a variety of cytokines, chemokines, and growth factors that may control barrier homeostasis. Furthermore, the capacity of keratinocytes was dysregulated in afflicted skin [29].
Little information has hitherto been available about circulating APRIL levels with ocular involvement in BD patients. Our study results showed that serum APRIL was significantly higher in our BD patients than controls. Meanwhile, serum APRIL levels were significantly higher in patients with ocular involvement than in patients without ocular involvement. This is in line with the work of Gheita et al., who found significantly higher serum APRIL concentrations in their BD patients than in their controls, and their levels were clearly elevated in those with panuveitis [9]. In contrast, Shaker et al. [22] found no significant difference in blood levels of BAFF, APRIL, and BCMA between patients with and without ocular disease, indicating that these TNF family members have a small role in the pathophysiology of BD visual symptoms.
In the current study, there were statistically significant associations between higher APRIL level and development of uveitis, cataract, and hypopyon. The most likely explanation is that serum APRIL level might have a role in the underlying inflammatory processes that contribute to these conditions; hence, it could potentially be used as a biomarker for predicting or monitoring the development of these eye-related complications in Behçet’s patients.
Although there were previous reports that investigated the role of APRIL in BD, and APRIL (and BCMA) might contribute to the pathogenesis of BD as well as by collaborating with other inflammatory cytokines to promote the activation and differentiation of effecter immune cells, it is worth noting that APRIL presents an attractive strategy for targeted intervention.
Our study showed that cutaneous involvement and arthritis were strong independent predictors for the development of ocular involvement in BD patients, which is a novel finding in this study, which requires more verification. To the best of our knowledge, no other studies have investigated this link before. Meanwhile, BD disease activity measured by BDCAF had no significant association with ocular manifestations. These data may suggest that serum APRIL might not be a reliable indicator of BD disease activity.
Study limitations
The current study’s limitations were its relatively small sample size and the fact that it was conducted in a single center. More research with larger sample sizes is required to corroborate our findings and address other risk factors of ocular involvement in BD.
Conclusions
We found that serum APRIL levels were markedly elevated in BD patients, particularly those with ocular manifestations, compared to both BD patients without ocular involvement and healthy individuals. The overexpression of APRIL in BD patients, particularly in terms of uveitis, cataract and hypopyon, supports the notion of a critical role for B cell activation factors in BD. These data may suggest that serum APRIL concentrations may distinguish a clinical subset of BD patients with ocular involvement. As a result, finding a new therapeutic strategy targeting the APRIL pathway might be beneficial in BD patients with ocular involvement. In the future, it might be possible to apply antibody-directed neutralization of APRIL not only in treatment of IgA nephropathy but also in BD. Current research in a mouse disease model and humanized anti-APRIL antibody VIS649 in nonhuman primates points to a potential therapeutic option. Especially, a strategy targeting the APRIL pathway might be beneficial in BD patients with ocular involvement.