Introduction
Rheumatoid arthritis (RA) is a chronic disease characterized by the presence of autoantibodies such as rheumatoid factors (RF) and anti-citrullinated protein antibodies (ACPAs) [1], which is thought to be experienced by 1% of the world population and 0.1–0.6% of the Indonesian population [2, 3].
Rheumatoid arthritis is related to bone loss, which could affect patients’ quality of life. The pathogenesis of bone loss is associated with inflammation and autoimmunity. Autoimmunity is particularly related to ACPA positivity. Multiple ACPA examinations are available nowadays, including synthetic CCP and anti-MCV [4, 5].
Multiple pathways of ACPA can affect bone remodeling dynamics. Anti-citrullinated protein antibodies affect bone destruction through multiple pathways such as osteoclast precursor activation, immune complex generation, increased production of receptor activator of nuclear factor-κB ligand (RANKL), and pro-inflammatory cytokine production [4, 6].
In contrast, ACPA also affects bone formation by producing the anti-inflammatory cytokine interleukin 27 (IL-27) and suppressing T helper 17 (Th-17) production [7, 8].
Therapy for RA, such as disease-modifying antirheumatic drugs (DMARDs), can affect bone loss by reducing inflammation and autoimmunity. Reduced inflammation can be seen as an improvement in disease activity that will be continued by reduced autoimmunity, as can be seen in the seroconversion of ACPA-positive to ACPA-negative. Finally, seroconversion of ACPA is related to bone loss reduction [9].
Disease Activity Scoring in RA, such as Disease Activity Score 28 (DAS28), is sufficient to express inflammation. Thus, disease remission and low disease activity (LDA) are associated with diminished or low levels of inflammation.
However, this scoring lacks the ability to measure autoimmunity because it uses non-specific inflammatory markers such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Thus, we cannot predict bone loss caused by autoimmunity through DAS28 scoring [10].
To measure the autoimmunity level, bone turnover markers (BTM) have been used as an alternative. Bone turnover markers are markers of bone destruction and bone formation. There are plenty of BTM available today, but the International Osteoporosis Foundation (IOF) recommends the use of C-terminal cross-linking telopeptide of type I collagen (CTX-1) as a destruction marker and N-terminal pro-peptide of type 1 procollagen (P1NP) as a formation marker [2, 11].
As mentioned above, patients with RA experience bone loss related to autoimmunity. Therefore, by using bone turnover markers (CTX-1 and P1NP) in patients with RA, we can measure the effect of autoimmunity on bone loss.
This study aimed to analyze the correlation between anti-MCV and CTX-1 and P1NP levels. Anti-MCV was chosen as a representative of anti-citrullinated protein antibodies in this study since anti-MCV has a high prevalence in Indonesian patients and is the most bone-reactive ACPA [12, 13].
Material and methods
The study was conducted in Rheumatology Clinics, Cipto Mangunkusumo National Hospital, from August to September 2019. The minimum number of samples calculated with the formula for number of samples for correlation research (considering an α of 0.05, the power of test 1-β being 0.90, and the correlation coefficient being set at 0.5) was 38.
We then selected 38 patients who met the criteria of inclusion and did not fulfill the exclusion criteria. The inclusion criteria were RA patients diagnosed with the ACR/EULAR diagnostic criteria for RA. The exclusion criteria were steroid use of more than 7.5 mg of prednisone, history of fractures, moderate or high disease activity, use of biological agents for RA treatment, and menopause or andropause.
We collected demographic data such as age, gender, body weight, height, disease duration, and menopausal or andropause symptoms. We defined menopause as not having any menstrual cycle in the last 12 months and defined andropause as three or more positive symptoms in the Androgen Deficiency in Aging Male (ADAM) questionnaire [14, 15].
Disease activity was measured using the DAS28 score, and treatment history was categorized into the use of methotrexate (MTX), non-MTX, and a combination of DMARDs.
All included patients then underwent laboratory examination for anti-MCV, CTX-1, and P1NP. The examination of anti-MCV, CTX-1, and P1NP was obtained by ELISA in the faculty laboratory in Jakarta, Indonesia. We categorized the patient as anti-MCV positive if the patient had an anti-MCV titer of > 10 IU/ml and negative if the patient had an anti-MCV titer ≤ 10 IU/ml [16].
There are no certain “cut-off values” for CTX-1 and P1NP levels that can be considered normal or increased since this marker has no positive or negative value and varies among populations [17, 18].
Statistical analysis
Descriptive analysis was performed using the mean and standard deviation (SD) for quantitative variables with a normal distribution. The median and range were used for variables with abnormal distributions. Correlation was analyzed using Spearman correlation.
The Mann-Whitney U test was used to compare means for data with abnormal distribution. A p-value of less than 0.05, one-sided, was considered statistically significant. All statistical analyses were conducted using SPSS statistical software (IBM version 20.0; SPSS Inc., Chicago, IL, USA).
Bioethical standards
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and adheres to the principles outlined in the Guideline for Good Clinical Practice International Conference of Harmonization (ICH) Tripartite Guideline (January 1997). The local ethics committee of the Cipto Mangunkusumo Institutional Review Board approved the study protocols.
Results
A total of 38 patients with RA were enrolled in this study. The baseline characteristics of the patients are shown in Table I. Thirty-four of our subjects were women (89.5%) with a mean age of 40 ±7.6, and the median disease duration was 36 (4–228) months. To control for the effect of inflammation on bone loss, we chose patients in remission and LDA.
Table I
Baseline patients’ characteristics
In this study, we found that 31 (81.6%) patients were in remission and seven (18.4%) patients were in LDA. Most patients were treated with MTX (60.5%).
The median level of anti-MCV was 54.5 IU/ml. Twenty-six of the patients were anti-MCV positive (68.4%). The median level of CTX-1 was 2.44 ng/ml and the median P1NP level was 0.047 ng/ml.
Anti-mutated citrullinated vimentin and C-terminal cross-linking telopeptide of type I collagen correlation
The authors correlated anti-MCV and CTX-1 levels with Spearman’s correlation and found r = 1.010 (p = 0.274). The correlation between anti-MCV and CTX-1 is shown in Figure 1.
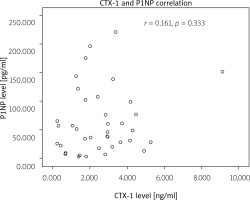
Fig. 1
Correlation between anti-mutated citrullinated vimentin and C-terminal cross-linking telopeptide of type I collagen.
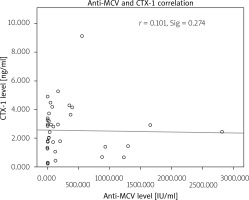
We found one outlier at the anti-MCV level, with a score of 2,813 IU/ml. This specific value belongs to a 42-year-old woman with a disease duration of 72 months and the use of a DMARD combination to control her disease.
This patient can be considered a patient with persistent anti-MCV positivity that does not convert after prolonged use of DMARD. However, CTX-1 level was not at the extreme level (2.470 ng/ml).
Anti-mutated citrullinated vimentin and N-terminal pro-peptide of type 1 procollagen correlation
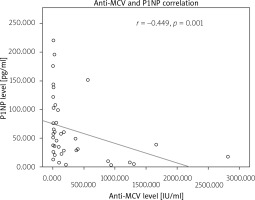
The authors correlated anti-MCV and P1NP levels with r = −0.449 (p = 0.001). The correlation between anti-MCV and P1NP is shown in Figure 2.
C-terminal cross-linking telopeptide of type I collagen and N-terminal pro-peptide of type 1 procollagen correlation
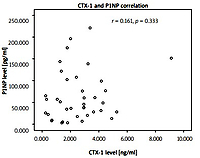
We also correlated CTX-1 and P1NP levels, with r = 0.161 (p = 0.333). The correlation between CTX-1 and P1NP can be seen in Figure 3.
Mean difference
We compared means using the Mann-Whitney U test to measure the mean difference of CTX-1 and P1NP according to anti-MCV status. There was no significant mean difference in CTX-1 levels between anti-MCV positive and negative patients (p = 0.312), but there was a significant mean difference in P1NP level between anti-MCV positive and negative patients (p = 0.019).
Discussion
In this study, we aimed to demonstrate how autoimmunity in RA can affect bone loss. Many studies have shown that ACPA is capable of inducing bone loss through several mechanisms [16–19].
To better understand how ACPA as an autoantibody affects bone loss, we chose patients with LDA and remission to avoid the effect of inflammation. This study included 38 patients with 31 (81.6%) patients in remission and 7 (18.4%) in LDA.
In Indonesian patients, conventional synthetic DMARDs such as methotrexate, leflunomide, sulfasalazine, and hydroxychloroquine are the most common drugs used to achieve disease remission.
In our study, methotrexate was administered to 23 (60.5%) patients to achieve remission or LDA. Conventional synthetic DMARD, particularly methotrexate, gives faster remission for ACPA-positive RA patients than ACPA-negative RA patients [20].
This DMARD is even capable of inducing immunological remission, that is, seroconversion of ACPA-positive to ACPA-negative patients [21]. Thus, this drug could affect autoimmunity-induced bone loss by converting autoimmunity status.
The finding of no correlation between ACPA level and CTX-1 level shows the heterogeneity of disease progression. In this study median disease duration is 36 (4–228) months; thus most of our patient are in the phase of established RA [22].
A patient with established RA has a different disease process than early RA in terms of the amount of pro-inflammatory cytokines and tissue destructive material. High ACPA levels in early RA are associated with bone destruction parameters such as cartilage oligomeric matrix protein (COMP) and matrix metalloproteinase 3 (MMP-3) [15].
In ACPA-positive-established RA patients and already in remission or LDA after csDMARD the disease process might have been modified by the presence of anti-inflammatory cytokines such as IL-27 and Th-17 cell suppression [8, 23].
In the presence of anti-inflammatory cytokines, ACPA is not capable of cytokine-induced osteoclast activation and thus bone destruction is partially inhibited and CTX-1 levels are not increased.
The presence of other autoantibodies such as rheumatoid factor (RF) and ACPA may affect CTX-1 levels. Anti-citrullinated protein antibody positivity is often accompanied by positivity of RF and ACPA [15].
In this regard, low levels of ACPA might be accompanied by high levels of other autoantibodies and vice versa; thus, the ACPA correlation with CTX-1 is not linear. This finding was also supported by the lack of a mean difference between CTX-1 levels in terms of ACPA status.
The correlation between ACPA and P1NP levels is moderate and negative; that is, a decrease in ACPA level may be associated with a moderate P1NP level increase. This is shown when inflammation is controlled and autoimmunity is suppressed, bone formation could take place. The inhibition of bone formation at low levels of ACPA might be related to Wnt pathway inhibitors, such as Dickoff-1 protein (DKK-1) or osteocalcin.
We also analyzed the correlation between CTX-1 and P1NP levels and found no significant correlation between these two variables. This result can be expected, as CTX-1 is a biomarker for bone resorption, while P1NP is a biomarker for bone formation [2].
Conclusions
Anti-citrullinated protein antibody levels are not linearly correlated with CTX-1 levels in RA patients with LDA and remission due to disease heterogeneity and treatment effects. The correlation between ACPA and P1NP is moderate, indicating the role of autoimmunity suppression in bone formation.
Thus, in addition to disease activity monitoring in RA patients, bone loss monitoring is recommended, even in patients with LDA and remission, to adjust the therapeutic continuum.