Introduction
Rheumatoid arthritis (RA) is a chronic systemic auto-immune disorder characterized by synovial inflammation, cartilage damage, and bone erosion associated with extrajoint involvement [1]. The disease has an estimated prevalence of 1.0% worldwide, negatively impacting quality of life and life expectancy [2]. In Colombia, a prevalence rate of 1.49% has been established [3].
Several studies have focused on the possible identification of early markers that indicate the onset and/or progression of the disease, joint damage, and the therapeutic response [4, 5]. Current findings have shown the role of fatty tissue as a pleiotropic organ specialized in endocrine functions and capable of controlling multiple pathophysiological processes, including inflammation, which has led to the recognition of the critical role of adipokines in the pathogenesis of RA [4, 5].
Adiponectin and leptin have recently been considered important factors in the pathogenesis of RA. These adipokines have a well-known metabolic function and exert an impact on the immune system. Adiponectin has shown pro-inflammatory functions at the joint level based on its ability to stimulate the secretion of inflammatory mediators instead of the anti-inflammatory effect described in diseases such as diabetes, atherosclerosis, and metabolic syndrome [6]. Leptin is a pro-inflammatory factor that stimulates the innate and acquired immune response, and its concentration increases during infection and inflammation [7].
A study evaluated the presence of leptin in patients with early rheumatoid arthritis (eRA) by observing increased levels of leptin, which were associated with the diagnosis of eRA (odds ratio [OR]: 2.95; CI: 1.54–5.07). Likewise, high levels of adiponectin in eRA were associated with a lower probability of having a modified Health Assessment Questionnaire (mHAQ) > 3, a BMI > 25, and a Routine Assessment of Patient Index Data 3 (RAPID3) > 12 (OR = 0.16; CI: 0.03–0.72). In patients with eRA, a greater likelihood of simultaneously developing high levels of leptin and interleukin 6 (IL-6) and low levels of adiponectin was observed (OR = 5.03; CI: 1.05–24.0) [8].
Furthermore, a higher frequency of obesity was identified in first-degree relatives based on BMI measurement [9], and high leptin levels were pinpointed after adjusting for clinical and demographic variables [8, 9]. Based on the aforementioned observations, adipokines and overweight/obesity could play a relevant role in diagnosing and predicting RA by facilitating its identification and optimizing the diagnosis promptly. All the above could impact clinical outcomes, which directly impact public health costs and quality of life.
The present study evaluated adipokines in patients with eRA (less than two years of evolution) and their correlation with disease activity; further factorial analysis was performed to explore these findings. Additionally, we established a cut-off point for each adipokine in the population compared with healthy controls as a proposal for use as a diagnostic biomarker.
Material and methods
Population
Patients older than 18 and younger than 65 years under the 2010 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria were included [10]. Patients were selected in the outpatient office for early disease (defined as patients with RA disease duration of less than 2 years) [11]. Patients diagnosed with type 2 diabetes mellitus, cancer, infectious disease, other autoimmune or autoinflammatory disease or taking lipidlowering medication were excluded at the time of selection. A control group with individuals selected from rheumatology consultation with mechanical rheumatism at the Institution or geographical area, matched by gender and age (maximum difference of one year), and verified as not having a personal or family history of autoimmune diseases, was formed.
Patients and controls were evaluated by a rheumatologist to identify demographic, clinical, and laboratory data. Clinimetric evaluation included joint count, Visual Analogue Scale for Pain (VAS Pain) [12], Disease Activity Score 28 (DAS28) [13], Simplified Disease Activity Index (SDAI) [14], RAPID3 [15], MHAQ [16].
All individuals accepted and signed the informed consent approved by the institutional ethics committee with HMC code 2016-041.
Laboratory methods
Leptin and Vaspin Quantification: Enzymatic Method (Diasource, KAP2281/MyBioSource, MBS267502)
The principle of the assay was based on the enzyme-linked immunosorbent assay (ELISA) technology. An anti-human antibody that targets leptin or vaspin was used to precoat the 96-well plates, and an anti–human biotin-labeled conjugated antibody targeted the adipokine in question. Serum samples, standards, and biotin conjugate were added to the wells and subsequently rinsed with a wash buffer. Streptavidin bound to horseradish peroxidase (HRP) was added, and excess material unbound from the reaction was removed.
Finally, a tetramethylbenzidine (TMB) substrate was used to visualize the horseradish peroxidase-linked enzymatic reaction. Horseradish peroxidase catalyzes TMB to produce a blue product that changes to yellow upon adding an acid stop solution. The yellow color’s optical density (OD) was proportional to the amount of human adipokine captured in each sample on the plate. The absorbance at 450 nm was read on a microplate reader, and the adipokine concentration was calculated.
Quantification of adiponectin, resistin, adipsin (MILLIPLEX MAP, HADK1MAG61K03)
Based on Luminex xMAP technology, which uses techniques to color-code microspheres using two different fluorescent dyes. Through precise concentrations of these dyes, distinctly colored beads are coated with a specific capture antibody. A biotinylated detection antibody is performed after the samples are exposed to the coated beads. The reaction mixture was then incubated with streptavidin-linked phycoerythrin-labeled conjugate, which was considered the reporter molecule, to complete the reaction on the surface of each microsphere. Finally, the data were acquired and analyzed in the Luminex 200 system.
For the quantification of C-reactive protein (CRP) and IL-6, chemiluminescence technology was used (Immulite 1000, Siemens Cat.-LKP1 No.10381411, 10286287, Gwynedd, United Kingdom) with a detectable value > 3.4 pg/ml for IL-6 and with CRP levels > 3 mg/l considered a high value. Quantitative measurement of anti-cyclic citrullinated peptide antibody (ACPA) IgG/IgA antibodies in serum was performed by ELISA (Quanta lite CCP 3.1 IgG/IgA, INNOVA Diagnosis; positive ≥ 20 IU/ml). Rheumatoid factor (RF) was measured using the kinetic turbidimetric technique (Spinreact, 110705; positive ≥ 20 IU/ml), and quantitative capillary photometry technology was used to measure the erythrocyte sedimentation rate (ESR) (Alifax Spa, Padua, Italy; normal value < 20 mm/h). All tests were performed following the manufacturer’s instructions.
Statistical analysis
Measurements of serum levels of adipokines (adiponectin, resistin, adipsin, vaspin, and leptin) were taken in patients with eRA and clinical and paraclinical disease activity indices. Then, a database was built using the statistical software package SPSS V26 for Windows. An exploratory data analysis was carried out with graphical methods, measures of central tendency, dispersion, and frequency distributions. Statistical assumptions were also evaluated to identify non-parametric vs. parametric tests to choose the statistical method with relevant indicators. Comparisons between groups of eRA and controls were performed using the χ2 test or Fisher’s exact test for categorical variables and the t-test or Mann-Whitney U test for continuous variables. Given the characteristics of the variables of interest (adipokines values), an evaluation was conducted using Spearman’s correlation coefficients and the Mann-Whitney U test.
A factorial analysis was performed using principal components analysis (PCA) to evaluate the dimensions involved in the results from the bivariate analysis. The PCA included rotation (Varimax) verified by the coefficient of conversion of rotating components (CCRC) with Kaiser normalization. To determine the factors, the sample size was verified by the Kaiser-Meyer-Olkin (KMO) statistical test, considering it adequate when the value of the coefficient was more significant than or equal to 0.6. Likewise, the Bartlett sphericity test (BST) was performed to validate the level of connection of the matrix with a confidence level of 95%.
The PCA models were established by BMI, adipokine levels, and disease activity indexes. The results are shown in graphs of loads of the solution rotated on a Cartesian plane and are expressed according to the contribution of each variable to the component that groups it, defined by the factorial load coefficient (FLC), whose values range between –1.0 and +1.0. A high contribution was considered when the FLC values were > 0.7, intermediate when they were between 0.5 and 0.7, and low when they were < 0.5. All FLC values less than 0.3 were excluded. Similarly, an analysis of the area under the curve was considered to identify the cut-off points to establish each adipokine’s value concerning eRA.
Results
Fifty-one patients with eRA and controls were included. Clinical and demographic characteristics are presented in Table I. Women represented 80.39% (n = 41) and median age was 52 (IQR 41–56). BMI was 25.12 ±3.8 kg/m2, overweight was present in 51%, obesity in 13.7%, hypercholesterolemia in 36.8%, and hypertriglyceridemia in 64.7%. Seropositivity for RF was 62.7%, for ACPA was 45.1%, and seropositivity for both RF and ACPA was 33.3%. The inflammatory marker CRP was positive in 56.9% and ESR was elevated in 29.4%.
Table I
Characteristics of patients with early rheumatoid arthritis and controls
[i] ACPA – anti-cyclic citrullinated peptide antibody, BMI – body mass index, CRP – C-reactive protein, DAS28 – Disease Activity Score of 28 joints, eRA – early rheumatoid arthritis, ESR – erythrocyte sedimentation rate, HLA – human leukocyte antigen, IgG – immunoglobulin G, mHAQ – modified Health Assessment Questionnaire score, RF – rheumatoid factor, SD – standard deviation, TNF – tumor necrosis factor.
Regarding disease activity, average VAS was 4.0 ±2.9, and the activity level measured by the DAS28 was 3.7 ±1.5 when calculated with ESR. Furthermore, in the DAS28 categories, 27.5% were in remission, 9.8% with low activity, and 62.8% with moderate or high activity. In addition, the SDAI calculation was 13.1 ±10.4 for the cases, with 58.8% showing moderate-high activity, 29.4% showing low activity, and 11.8% in remission. An analysis of the mHAQ found a score of 2.7 ±3.2 among the cases, with maximum disability at 39.2% and disability-free at 39.2%; the remaining 21.6% had an intermediate value (Table I). Among patients with an eRA diagnosis, 80.4% received disease-modifying therapy (methotrexate was used in 78.4% and anti-TNF therapy in 2% of the patients). The use of corticosteroids was reported in 47.1% of patients.
Adipokine level correlation analysis and principal component scores
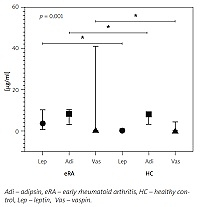
The adipokine levels in the studied population are detailed in Table II. Bivariate analysis showed that resistin levels were higher in patients with RAPID3 near remission (p = 0.041), and levels of adiponectin, vaspin, and leptin were lower in patients with DAS28–ESR in remission (p = 0.033, p = 0.012 and p = 0.017, respectively); all other variables analyzed were found to be non-significant. Correlation analysis showed a significant association between adipsin levels and BMI (r = 0.096, 95% CI: 0.014–0.523) and the RAPID3 activity index (r = 0.295, 95% CI: 0.021-0.527). Vaspin and leptin levels also correlated with BMI (r = 0.297, 95% CI: 0.020–0.531 and r = 0.409, 95% CI: 0.151–0.615, respectively); median serum concentration for these adipokines are presented in Figure 1. There were no significant differences in analyzing total cholesterol and triglycerides with adipokines.
Table II
Receiver operating characteristic curve analysis of serum adipokine levels in patients with early rheumatoid arthritis
Fig. 1
Adipsin, vaspin, and leptin levels between eRA and HC groups.
Adipsin, vaspin, and leptin levels were reported in μg/ml in the median and interquartile range for eRA and HC groups; comparisons between groups are statistically significant.
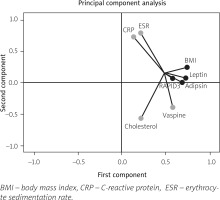
The PCA scores were calculated, and each score was weighted for the relative contribution to the explained variance. The model was established with a KMO test of 0.6 with a p-value < 0.001. The first component merged the influence of adipsin (0.682) and leptin (0.725) together with BMI (0.740) and disease activity index RAPID3 (0.576) in eRA patients. Additionally, the second component reported the inverse influence of vaspin (–0.396) on cholesterol levels (–0.565) and positively with ESR (0.793) and CRP (0.728). Additional variables had non-significant weight in the components analyzed. Graphical analysis of components in factorial load coefficients is presented in Figure 2.
Fig. 2
Principal components analysis (PCA) of adipokines and activity indices.
The PCA for continuous variables in two components. In this case, component 1 (Comp-1) represents adipokines and disease activity, and component 2 (Comp-2) represents inflammatory markers. The figure shows the variables in a vector map as a geometric representation of variables presented by lines where the length expresses the standard deviation of each variable, and the angles among variables show their correlations (acute angles represent stronger correlations, right angles no correlations, and straight/obtuse angles show inverse correlations). Note how adipokines adipsin and leptin with BMI and RAPID3 as disease activity index are grouped in a network of acute angles showing standard behavior in Comp-1. However, Comp-2 had a strong correlation between ESR and CRP with an inverse correlation with cholesterol levels and vaspin.
Adipokine receiver operating characteristic curve analysis
Receiver operating characteristic (ROC) curve analysis was performed to evaluate the diagnostic accuracy of adipokines in discriminating patients with eRA from control subjects. Leptin tended to be more specific (AUC was 0.812 with 74.5% specificity and a positive likelihood ratio [LR+] 3), followed by adipsin (AUC was 0.586 with 86.3% specificity and LR+ 2.9), and vaspin (AUC was 0.692 with 62.9% specificity and LR+ 1.9). Next, we investigated cut-offs for the different biomarkers, as described in Table II.
Discussion
We have not identified studies in Latin American countries other than those published by our group in adipokine profiles [9, 17]. This analysis identified a statistically significant association between adipsin and disease activity, validated with PCA and bivariate analysis with DAS28–ESR and RAPID3 in patients with eRA.
For example, in Egypt, three studies have been carried out on adipokine levels in eRA (less than one year); El-Hini et al. [18] reported that visfatin statistically correlated with the lipid profile (cholesterol, triglycerides, and LDL). There was a positive correlation with an insulin resistance marker – the homeostasis model assessment (HOMA-IR) [18]. Our correlation analysis showed no significant differences in total cholesterol and triglycerides. However, the PCA revealed an association with cholesterol and vaspin and an inverse association with acute phase reactants. Ismail et al. [19] reported a correlation between leptin and the disease activity indices measured by DAS28.
Meanwhile, El-Barbary et al. [20] found that resistin levels were significantly higher in patients with eRA and positively correlated with disease activity variables. A previous study in our institution identified high leptin levels associated with eRA diagnosis, with an OR of 2.95 and a 95% CI: 1.54–5.07 [9]. Within the studied population, resistin levels were higher in patients with RAPID3 near remission, and adiponectin, vaspin, and leptin were lower in patients with DAS28–ESR in remission. RAPID3 had a weak correlation with adipsin, and the PCA showed the relation of adipsin and leptin with RAPID3, as reported by other groups [19–21].
Furthermore, in a Swedish population with RA (less than two years of evolution), decreased levels of leptin and adiponectin were identified in smokers, and an association between the activity evaluated by DAS28, a high BMI, and the female gender was also identified [21]. Despite a smoking frequency of 33.3%, there were no associations between adipokine concentrations and cigarette smoking in our population.
Vaspin has been identified as an adipokine associated with RA development in healthy individuals with RF and/or positive ACPA [22]. In patients with established RA, its presence had been correlated with the duration of the disease [23]. In this study, elevated levels of vaspin correlated with the RAPID3 score; however, although the exact correlation was not identified in the DAS28, this marker could be considered in patients experiencing elevated vaspin levels of RA in its early stages.
El-Barbary et al. [20] observed that serum levels of TNF-α and resistin decreased with the use of methotrexate; when combined with atorvastatin, the decrease was even more significant. Similar findings were reported in Spain, with a decrease in adiponectin and resistin with an increase in leptin [24]. In Sweden, the Engvall group and collaborators provided no evidence of the influence of biological therapy in a two-year follow-up on adipokine levels [25]. In our study, no correlation was identified with patients’ type of treatment.
Currently, disparities exist regarding the definition of the time from diagnosis and the onset of symptoms to diagnosis. The selected time was less than two years, although other definitions use less than one year [18–20, 24–29] and even less than six months in other populations [30, 31]. The recent findings could lead to considering the measurement of adipokines as an early marker associated with the activity. However, considering the delay in receiving care and patients’ access to rheumatology services in our health system, we consider the timeframe of two years to be appropriate.
Additionally, this study analyzed the cut-off point of these cytokines in patients with eRA, given that this value has not been defined in the Colombian population from controls; for this reason, it is worth highlighting the values to identify a more sensitive value for the five measured adipokines. Adiponectin required the highest value to achieve moderate sensitivity, with a specificity of only 51%, with similar results to resistin.
Study limitations
One of the limitations associated with this study was its design, which does not allow for an interpretation of the causality of the results; however, the prospective follow-up with these individuals will provide relevant data related to eRA and the relationship with adipokines.
Another limitation was the small number of patients due to difficulties recruiting patients experiencing the early stages of the disease and the strict study inclusion criteria. It is important to continue testing patients with early-stage RA to determine the nature of such correlations because the magnitude was low, but additionally, PCA analyses support these results. However, it is worth emphasizing that all our patients received conventional treatment. One of this study’s strengths is that, to our knowledge, it is one of the first to analyze a broad array of adipokines in patients with eRA in the Latin American population and has validated findings from a study previously carried out by our group [19]. Given that we did not identify another similar study of a Colombian population, the results of the cut-off points provide essential information to be analyzed in subsequent studies.
Further studies could include a proposal for carrying out predictive models regarding the response to pharmacological treatment or studies that aim to validate these findings by measuring other biomarkers related to inflammation and disease activity.
Conclusions
Adipokine levels are relevant in eRA, especially with disease activity indexes. Resistin levels were higher in patients with an activity index near remission. Otherwise, adiponectin, vaspin, and leptin levels were lower in patients with low activity indexes. RAPID3 correlated with adipsin. This study reported the correlation and cut-off points of adipokines based on BMI and disease activity levels in patients with eRA. Additional studies are required to determine the role of adipokines as potential biomarkers in this group of patients.