Introduction
The α-antiphospholipid syndrome (APS) is a systemic, autoimmune disease of unknown etiology characterized by arterial or venous thrombosis and/or recurrent miscarriage coexisting with the presence of antiphospholipid antibodies (aPL). Lupus anticoagulant (LAC), moderate to high titers of anticardiolipin (aCL) or anti-β2-glycoprotein I (aβ2GPI) antibody detection in the serum twice in a 12-week period confirms the diagnosis [1]. It can occur either as a primary disorder or accompanying other systemic autoimmune diseases, mainly systemic lupus erythematosus (SLE). Damage caused by APS is mainly driven by thrombosis, which can affect any organ. Twenty percent of strokes under 40 years old occur in APS as well as up to 25% of all fetal losses [2]. Deep vein thrombosis and subsequent post-thrombotic syndrome have significant adverse effects on patients’ life quality [3, 4]. It has been demonstrated that patients with post-thrombotic syndrome have higher quality of life (QoL) impairment than those without [5].
Raised awareness and improved understanding of APS have led to higher survival of patients with this condition over the past decades. At the same time, APS manifestations may cause numerous restrictions in psychological functions – anxiety, depression, family conflicts, social isolation, and limitation in workability. Finally, working inability increases anxiety and depression due to financial problems [6]. Unexpected symptoms such as stroke or pulmonary embolism acquired damage (post-thrombotic syndrome, paresis, migraine, seizures, etc.), pregnancy losses and uncertain prognosis lead to depression and despair (hopelessness) [7].
Health-related QoL (HRQoL) can be described as a multi-dimensional designation and refers to objective living conditions as well as to the subjective feeling of well-being. The HRQoL does not only depend on the underlying disease, because functioning and emotional well-being can differ between individuals living with the same disease [8]. Whether modifiable factors such as social support, fatigue, helplessness, and other illness-related behaviors have an impact on HRQoL in APS remains underexplored [9, 10]. Numerous studies have investigated HRQoL in patients with other autoimmune diseases – rheumatoid arthritis, SLE, Sjögren disease, or systemic sclerosis. The findings revealed differences between autoimmune diseases and support the need for further investigations. At the same time, examination of APS itself is difficult due to coexistence of multiple autoimmune diseases [11, 12].
The clinical and biological parameters currently used to guide treatment in patients with APS may be less important to patients than general health perception, vitality, energy, fatigue, and social roles and activities [13]. Therefore, measuring HRQoL in clinical practice can improve patients’ compliance and adherence. Health-related quality of life should be considered as an additional outcome measure to the traditionally performed activity and damage measures [14]. Instruments for the measurement of HRQoL should be specific for each disease and generic at the same time. Such instruments for APS are not available at the moment.
To date, most studies have focused on SLE [20–24], while primary APS patients have been analyzed less frequently [25–28]. We aimed to establish whether APS leads to life quality impairment and whether concomitant SLE has a different effect on the HRQoL.
The objectives of this study were: to evaluate HRQoL in APS patients by applying the 36-Item Short Form 36 Health Survey (SF-36) and World Health Organization Quality-of-Life Scale (WHOQoL-BREF); to examine the impact of primary APS and APS associated with SLE on patient HRQoL; and to provide a description of the APS patient population, including major issues associated with the condition.
Material and methods
Group characteristics
One hundred twelve patients with APS were included in the study, 57 of them with primary APS and 55 with coexisting SLE. These patients were followed at the Department of Connective Tissue Diseases, National Institute of Geriatrics, Rheumatology and Rehabilitation, Warsaw, Poland and enrolled in the present study between September 2013 and 2015. Antiphospholipid syndrome was diagnosed according to revised Sydney classification criteria for APS in every patient (Table I) [1]. Full medical history and physical examination data were recorded at inclusion for each patient. Baseline data included demographic information (age, sex, education, work activity), objectively documented APS (thrombotic and obstetrical events), multiple features associated with APS (heart valve disease, livedo reticularis, thrombocytopenia, nephropathy, superficial vein thrombosis, neurological manifestations, etc.), thromboembolic and cardiovascular risk factors, inherited thrombophilia, concomitant diseases, and current medication. All the patients underwent laboratory tests including antinuclear antibodies and aPL profile with LAC, aCL and a2GPI. Patients received stable treatment for APS/SLE. All patients completed questionnaires on QOL – SF-36 and WHOQOL-BREF. For comparison, 112 healthy controls matched by gender, age, and race were included.
Table I
Demographic data and clinical manifestations in study groups
Health-related quality of life measurement
Short Form 36 Health Survey
It is a reliable, generic, and valid measure for HRQoL assessment in the healthy population and many different diseases [14]. Short Form 36 Health Survey is a self-report questionnaire with demonstrated consistency and validity. It has been translated into different languages and transculturized in populations in various rheumatic diseases [15, 16]. Short Form 36 Health Survey is a specific tool to assess the quality of life through physical functioning, role physical functioning, bodily pain, general health, vitality, and social, emotional, and mental health [17]. It is composed of 8 domains measuring physical and psychological status: role physical (RP), general health (GH), bodily pain (BP), physical functioning (PF), role emotional (RE), vitality (VT), mental health (MH), and social functioning (SF). Six of the eight domains are scored on multipoint scales. Computation of scale scores is performed by same-scale item summation followed by transformation of the raw scale score on a range from 0 to 100 (from lowest to highest possible level of functioning) [18].
WHOQoL-BREF Quality of Life Scale
The WHOQoL-100 scale is multidimensional, developed for a wide range of psychological and physical disorders. Initially, the WHOQoL scale consisted of 100 questions about 24 aspects of quality of life. However, it was difficult for researchers to use, so WHOQoL-BREF was created [19]. The WHOQoL-BREF consists of four domains: physical health (PH; 7 items), psychological well-being (PS; 6 items), social relationships (SR; 3 items), and environmental health (EH; 8 items). All questions are rated on a 5-point Likert scale, and the item score is between 1 and 5. Raw scores in each domain were changed to a score of 4–20 based on the guideline. All domain scores vary linearly, from 0 to 100, where “100” indicates the highest possible QoL [27].
Statistical methodology
The statistical analysis was begun with a substantive and logical review of the collected data, and the Shapiro-Wilk test was performed to verify normality of distributions of quantitative variables. The results of quantitative variables with a normal distribution, or quantitative variables with a coefficient of skewness of less than 1.5, are shown as the arithmetic mean and standard deviation; in the case of irregular distribution, they are shown as the median and interquartile range. Nominal variables are presented as the count and relative frequency (%).
In the case of variables with normal distributions, significance of differences between mean values was estimated using Student’s t-test (comparison of 2 groups) and analysis of variance with Tukey’s post-hoc test (comparison of 3 groups). Variables with non-normal distribution were compared using the non-parametric Mann-Whitney test (2 groups) and non-parametric Kruskal-Wallis test (3 groups).
Significance of differences of 2 or 3 categorical variables was performed with the χ2 test corrected for continuity or the Yates-corrected Fisher’s exact test, depending on the number of observations. Verification of null hypotheses was conducted at a significance level of 0.05 with two-sided testing. All statistical analyses were performed using SAS statistical 9.2.
Bioethical standards
This analysis was an additional work on the subject “Pathogenesis and clinical picture of atherosclerosis in patients with SLE, MCTD and APS – serological, genetics and profile of cytokines influencing atherosclerosis plaque”.
Agreement of National Institute of Geriatrics, Rheumatology and Rehabilitation Bioethical Committee for all the study and sub-analysis (agreement from 12.12.2013) was obtained. All subjects gave informed consent for participation in the study.
Results
Patient characteristics at inclusion
Over 2 years, 112 patients were included. The mean age was 47 years (47.6 ±13.8), and 96 of them (85.7%) were women. All patients were diagnosed with APS according to diagnostic criteria from Sydney (Suppl. Table I) [1]. Fifty-five patients suffered from APS and SLE (SLE/APS), while the remaining 57 patients had primary APS. A total of 112 HRQoL questionnaires were collected. Patient baseline characteristics are described in Tables I and II. The mean SELENA-SLEDAI score for patients with SLE was 3.92.
Table II
Antiphospholipid antibodies’ presence in study group
Most of the patients had a history of thrombosis – arterial and venous thrombosis was numerically more frequent in the SLE/APS group than the APS group. Deep vein thrombosis was statistically significantly more frequent in the SLE/APS group (Table I). In both groups, pregnancy losses had occurred in about 40% of patients. Depression was diagnosed in approximately 10% of patients in both groups. The most frequent antiphospholipid antibody was LAC in both groups, followed by aCL immunoglobulin G (IgG) and aβ2GPI IgG (Table II).
Life quality in the study group
Life quality in the study group was assessed by WHOQoL-BREF and SF-36 (Table III).
Table III
Results from all domains in WHOQoL-BREF and SF-36
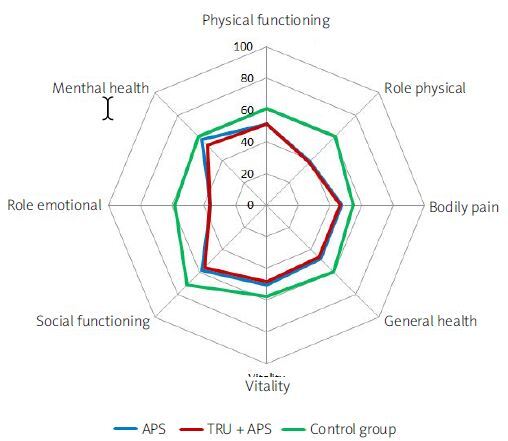
The overall HRQoL in APS and APS/SLE groups was lower than in controls both in mental and physical components of SF-36 (p < 0.0001). The only difference between the groups was observed in the mean mental health (MH) score, which was lower only in the APS/SLE group in comparison to controls (53.5 ±16.3 vs. 61.2 ±10.4 and 58.2 ±14.8, respectively). No difference in HRQoL was found between APS and APS/SLE groups in all domains (Table IV, Fig. 1).
Table IV
SF-36 domain differences between APS, APS/SLE and control group
| APS (1) | SLE/APS (2) | Control group (3) | p-value | 3 vs. 1 | 3 vs. 2 | 1 vs. 2 | |
|---|---|---|---|---|---|---|---|
| Physical function (PF) | 50.0 ±9.0 | 51.0 ±9.2 | 61.1 ±4.3 | < 0.0001*** | < 0.0001*** | < 0.0001*** | 0.999 |
| Role-physical (RP) | 39.0 ±3.3 | 38.0 ±3.0 | 61.1 ±5.0 | < 0.0001*** | < 0.0001*** | < 0.0001*** | 0.415 |
| Bodily pain (BP) | 47.6 ±11.9 | 46.5 ±10.1 | 54.8 ±8.2 | < 0.0001*** | < 0.0001*** | < 0.0001*** | 0.848 |
| General health (GH) | 47.9 ±10.2 | 46.7 ±9.0 | 60.0 ±8.5 | < 0.0001*** | < 0.0001*** | < 0.0001*** | 0.764 |
| Vitality (VT) | 50.5 ±15.1 | 48.4 ±15.1 | 57.8 ±10.1 | < 0.0001*** | 0.0021** | < 0.0001*** | 0.670 |
| Social function (SF) | 58.4 ±20.1 | 55.6 ±18.7 | 71.15 ±11.5 | < 0.0001*** | < 0.0001*** | < 0.0001*** | 0.618 |
| Role-emotional (RE) | 35.5 ±3.4 | 35.8 ±3.6 | 58.4 ±5.3 | < 0.0001*** | < 0.0001*** | < 0.0001*** | 0.954 |
| Mental health (MH) | 58.2 ±14.8 | 53.5 ±16.3 | 61.2 ±10.4 | 0.0027** | 0.370* | 0.0017** | 0.148 |
| Physical component | 43.7 ±7.9 | 43.7 ±7.5 | 56.2 ±5.8 | < 0.0001*** | < 0.0001*** | < 0.0001*** | 1.000 |
| Mental component | 49.4 ±13.6 | 46.2 ±14.0 | 59.9 ±9.9 | < 0.0001*** | < 0.0001*** | < 0.0001*** | 0.3372 |
WHOQoL-BREF and SF-36
The SF-36 mental component was associated with all domains of WHOQoL-BREF, while the physical component was associated only with physical health (p < 0.001; Table V).
Table V
Association between SF-36 and WHOQoL-BREF in whole study group
| APS + APS/SLE (n = 112) | Physical domain | Psychological domain | Social relationship domain | Environmental domain | ||||
|---|---|---|---|---|---|---|---|---|
| Pearson correlation coefficients | Spearman correlation coefficients | Pearson correlation coefficients | Spearman correlation coefficients | Pearson correlation coefficients | Spearman correlation coefficients | Pearson correlation coefficients | Spearman correlation coefficients | |
| Physical function (PF) | 0.60588*** < 0.001 | 0.62756*** < 0.0001 | 0.38507*** < 0.001 | 0.43485*** < 0.0001 | 0.35993** 0.0002 | 0.41291*** < 0.0001 | 0.36627*** 0.0001 | 0.43626*** < 0.0001 |
| Role-physical (RP) | 0.65510*** < 0.001 | 0.70675*** < 0.0001 | 0.4119** < 0.0011 | 0.46701*** < 0.0001 | 0.35728** 0.0002 | 0.41493*** < 0.0001 | 0.38749*** < 0.0001 | 0.44926*** < 0.0001 |
| Bodily pain (BP) | 0.62741*** < 0.001 | 0.59299*** < 0.0001 | 0.38335*** < 0.001 | 0.37314*** < 0.0001 | 0.29110** 0.0029 | 0.27180** 0.0055 | 0.37021*** 0.0001 | 0.35718** 0.0002 |
| General health (GH) | 0.55581*** < 0.001 | 0.52566*** < 0.0001 | 0.37208*** < 0.001 | 0.42525*** < 0.0001 | 0.27290** 0.0053 | 0.28376** 0.0037 | 0.27767** 0.0045 | 0.33306** 0.0006 |
| Vitality (VT) | 0.61677*** < 0.001 | 0.59594*** < 0.0001 | 0.51517*** < 0.001 | 0.56327*** < 0.0001 | 0.34406** 0.0004 | 0.39574*** < 0.0001 | 0.33458** 0.0006 | 0.39750*** < 0.0001 |
| Social function (SF) | 0.69829*** < 0.001 | 0.70847*** < 0.0001 | 0.53568*** < 0.0001 | 0.58276*** < 0.0001 | 0.50048*** < 0.0001 | 0.54218*** < 0.0001 | 0.45740*** < 0.0001 | 0.47844*** < 0.0001 |
| Role-emotional (RE) | 0.57285*** < 0.001 | 0.59454*** < 0.0001 | 0.48290*** < 0.0001 | 0.51478*** < 0.0001 | 0.47980*** < 0.0001 | 0.51689*** < 0.0001 | 0.36169** 0.0002 | 0.38801*** < 0.0001 |
| Mental health (MH) | 0.61036*** < 0.001 | 0.58797*** < 0.0001 | 0.68298*** < 0.0001 | 0.68988*** < 0.0001 | 0.58624*** < 0.0001 | 0.63951*** < 0.0001 | 0.52591*** < 0.0001 | 0.56299*** < 0.0001 |
| Physical component | 0.56191*** < 0.001 | 0.56302*** < 0.0001 | 0.22143** 0.0246 | 0.28394** 0.0037 | 0.16160* 0.1029 | 0.18345* 0.0636 | 0.24343* 0.0132 | 0.28810** 0.0032 |
| Mental component | 0.65261*** < 0.001 | 0.64012*** < 0.0001 | 0.65629*** < 0.0001 | 0.68221*** < 0.0001 | 0.55941*** < 0.0001 | 0.62378*** < 0.0001 | 0.48660*** < 0.0001 | 0.50285*** < 0.0001 |
In both study groups, mental component correlated with physical health, psychological well-being and social relationships. Physical health and environmental health correlated with almost all SF-36 domains in the APS group. At the same time, in the APS/SLE group the correlations between SF-36 and WHOQoL-BREF were weaker – mental health and the mental component correlated most strongly with physical health, psychological well-being and social relationships (Suppl. Tables II and III).
Impact of clinical variables on health-related quality of life impairment
Impact of age and sex
Age correlated with low scores in the physical and mental components in the APS group, but only with low scores in the physical component in the APS/SLE group. There was no association with gender in any SF-36 domain (Suppl. Tables IVA and IVB).
Impact of antiphospholipid antibodies on health-related quality of life impairment
The presence of LAC correlated with improved RP scores (p < 0.0001) in the primary APS group. Between the presence of aCL IgM and lower RP scores in APS/SLE group a moderate evidence was observed (p = 0.0443), but no association was found for aCL IgG. Weak evidence exist for aβ2GPI IgG presence and higher scores in multiple domains – PF (p = 0.0389 APS; p = 0.0213 SLE + APS) in both groups and RP (0.0423), VT (0.0291), RE (p = 0.0439), and MH p = 0.0067) in primary APS. In the presence of aβ2GPI IgG mental component was increased in the APS group (p = 0.0244), while for APS/SLE physical component was increased (p = 0.0332). There was no association between aCL IgG and aβ2GPI IgM and HRQoL (Suppl. Tables V A-E).
Impact of thrombosis on health-related quality of life impairment
Arterial and venous thrombosis both separately and altogether was not associated significantly with HRQoL impairment in the primary APS group. At the same time, a history of thrombosis was associated low scores in RP (p = 0.0016) and BP (p = 0.0092) domains and PH component (p = 0.0118) in the APS/SLE group. Interestingly, there was no significant association of arterial thrombosis – when venous thrombosis impaired multiple domains (PF, RP, BP, GH, VT) and significantly PH (p = 0.002) in the APS/SLE group (Suppl. Tables VIA, VIB).
Discussion
Antiphospholipid syndrome is a less frequent disease than SLE and often coexists with it. The HRQoL is routinely investigated in clinical trials of patients with SLE, so there are limited scientific data on APS.
In our study, similarly to previous ones, APS patients had a lower HRQoL in comparison with healthy individuals [24–27]. In a study by Zuilly et al. [25], higher HRQoL impairment was observed in APS patients between 45 and 54 years old compared with the general population as well as in women. In our study, HRQoL impairment associated with age in both groups, but not with gender.
No difference in HRQoL was observed between APS and APS/SLE groups in all domains. In comparison to healthy controls, both mental and physical components of SF-36 were lower in both study groups. Three other studies [25, 26, 28] observed in most of the SF-36 domains, especially physical ones, lower scores in APS/SLE groups compared with APS. Perhaps disease activity and organ damage may be responsible for the heterogeneity of the results in these studies.
In our study, we observed a strong association between improved RP domain and the presence of LAC in the APS group. Moderate evidence were found between the presence of aβ2GPI IgG (but not IgM) and improvement of some SF-36 domains. Mental component improvement was observed in the APS group and physical component in the APS/SLE group. It may suggest that the presence of aPL, although a risk factor of thrombosis, is not a reason for impaired HRQoL in APS patients. So far, two studies [25, 28] have evaluated aPL only in patients with SLE without a diagnosis of APS, but no study has evaluated type of antibody and its correlation with HRQoL impairment.
The question of whether the thrombotic history affects HRQoL in APS is still valid and very important. In our study, the history of thrombosis impaired the SF-36 domains only in the APS/SLE group, and it was only in patients with venous, not arterial events. It may be explained by the presence of post-thrombotic syndrome and the main chronic complication of pulmonary embolism is chronic thromboembolic pulmonary hypertension (CTEPH) [29]. In a study of patients with SLE and/or APS [28], a history of a previous thrombovascular event (TE) was associated with impaired mental and physical domains affecting their HRQoL. The physical component was more affected, and this effect was not related to the type of TE or the age of the patients. It is consistent with our findings. A previous study reported that arterial thrombosis was associated with lower scores in HRQoL of APS patients [16], but our study did not show it in any study group. The type of thromboembolic history influenced HRQoL in the general population – a history of either venous or arterial [30, 31] thrombosis is associated with impaired HRQoL assessed by the SF-36 [3–5]. Data coming from our study are not in line with other literature sources, and the results may depend on the type of arterial event, which differs among the studies.
Studies on HRQoL in patients with a history of thrombosis in patients with APS, SLE as well as APS associated with SLE (APS/SLE) [21, 22] proved that HRQoL is better for patients with primary APS compared to patients with APS and coexisting autoimmune disease [6]. Our study revealed that APS results in impaired HRQoL, and the concomitant SLE does not magnify this effect in all SF-36 domains. It may suggest that damage associated with SLE is not as burdensome as the impact of thrombosis or pregnancy loss on HRQoL.
The added value of this study is that it is the first, to our knowledge, reporting the association between SF-36 and WHOQoL-BREF domains. Especially the mental component was associated in the whole group with all WHOQoL-BREF domains. In a study by Huang et al. [32], these associations were weak when using both instruments, and they concluded that both SF-36 and WHOQoL-BREF appeared to measure different constructs – the SF-36 measured health-related QoL, while the WHOQoL-BREF measured global QoL. At the same time, in patients with HIV infection, both the WHOQoL-BREF and the SF-36 were reliable and valid health-related QoL instruments. Our observations showed, similarly to the study by Hsiung et al. [33], closer associations between these 2 scales for all analyzed patients, with stronger statistical significance in APS than APS/SLE. As widely discussed, SF-36 discriminates better among different levels of health status and utilization and may be more appropriate to describe health-related functioning and perceptions as objective QoL. At the same time, the WHOQoL-BREF may be more appropriate to measure self-reported subjective QoL [34]. In conclusion, objective and subjective HRQOL assessment in APS is more concordant in the APS/SLE group.
Limitations
There are several limitations to this study because environmental or emotional influences could not be controlled. Additionally, the sample in this study is not representative of all patients with APS. The majority of patients were Polish females, which prevents the generalization of findings to other nationalities and males. An additional aspect not covered in the present research is the influence of disease activity on HRQoL – mild to moderate SLE activity (mean SLEDAI 3.92) did not allow us to perform any detailed analysis on this topic.
Conclusions
Our study confirms that APS has a significant impact on patients’ HRQoL but there might be a varying effect on physical and mental HRQoL. This study confirms previous findings on HRQoL impairment compared to a healthy population. The impact of thrombosis needs further studies. Investigation of HRQoL characteristics in APS and specific evaluation of health interventions can lead to improvement of patients’ perception of quality of life. The health-related quality of life is an important part of comprehensive care of any chronic disease. Future approaches to treat patients with APS and SLE should include HRQoL improvement.