Dry eye disease (DED) is no longer only an ophthalmologic concern; it is increasingly recognized as a systemic signaling disorder that frequently intersects with autoimmune conditions encountered in rheumatology, particularly Sjögren’s disease and rheumatoid arthritis (RA) [1]. Traditionally, diagnosis relied on subjective questionnaires and clinical examinations. However, the past decade has witnessed the emergence of objective and now automated diagnostic technologies that have reshaped disease detection, subtyping, and therapeutic decision-making. This editorial highlights the diagnostic tools most relevant to rheumatologists who co-manage ocular surface disease alongside ophthalmology colleagues.
The TFOS DEWS III defined DED as a “loss of tear-film homeostasis” and proposed three core diagnostic metrics: non-invasive tear breakup time (NIBUT), tear osmolarity, and ocular surface staining [2]. These, combined with validated questionnaires such as Dry Eye Questionnaire – 5 item (DEQ-5) or Ocular Surface Disease Index (OSDI), offer 70–90% diagnostic sensitivity, even accounting for the frequent sign–symptom discordance observed in autoimmune patients [3]. The Schirmer test, though one of the oldest objective methods, lacks reproducibility and often underestimates evaporative disease. Distinguishing between evaporative (e.g., meibomian gland dysfunction) and aqueous-deficient (e.g., lacrimal infiltration) subtypes is critical both for tailoring therapy and for understanding the degree of autoimmune involvement [4].
Handheld micro-osmometers (e.g., TearLab, I-Pen) can quantify tear osmolarity from 50 nl of tears. A reading of ≥ 308 mOsm/l or an inter-eye difference greater than 8 mOsm/l is considered diagnostic. These tests are operator-independent, and a drop greater than 10 mOsm/l often correlates with symptom improvement [5]. However, reflex tearing during sample collection may compromise accuracy, variability remains high, and the cost, approximately €20 for bilateral testing, can hinder accessibility [6]. Moreover, specificity is reduced in very mild disease, and care must be taken to collect samples before the instillation of anesthetic drops [7].
InflammaDry detects elevated tear matrix metalloproteinase-9 (MMP-9 ≥ 40 ng/ml) within 10 min. Clinical trials have shown 85% sensitivity and over 90% positive predictive value in symptomatic patients [8]. This tool is particularly useful in identifying patients likely to benefit from immunomodulatory therapy, such as topical cyclosporine, and has demonstrated relevance in Sjögren’s and RA-associated dry eye [9].
Further advancement in diagnostic capability is provided by automated ocular surface imaging. Placido-disc topographers and dry-eye analyzers (e.g., Idra, cDiag, Keratograph 5M) allow fluorescein-free NIBUT measurement. A value below 10 s is diagnostic and offers greater repeatability than fluorescein tear breakup time (TBUT) while avoiding dye-induced artifacts. These systems also assess lipid layer thickness (LLT), tear meniscus height, and blink patterns [3]. High-resolution meibography (Fig. 1) provides detailed imaging of the meibomian glands, detecting gland atrophy frequently seen in systemic sclerosis and long-standing RA [10]. When Schirmer test results are normal but gland loss is evident, an evaporative pathogenesis is confirmed [2]. Such findings not only support diagnosis but also guide management: visual biofeedback from imaging improves adherence to warm compresses and lid hygiene routines, and marked gland dropout may favor thermal pulsation therapies over punctal plugs.
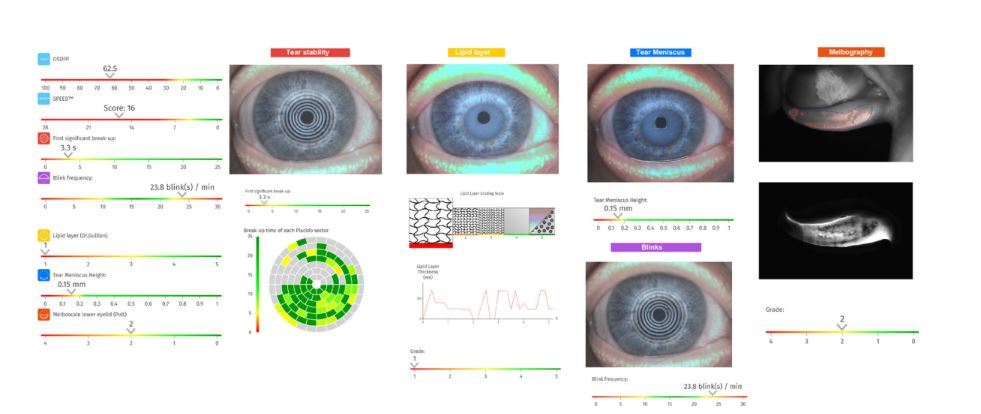
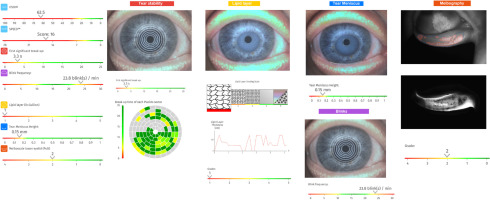
Fig. 1
Automated dry eye assessment of the right eye generated by the C.Diag platform (Quantel Medical) in a 45-year-old female with Sjögren’s disease and alopecia areata. Subjective symptom scores are high, with an OSDI of 62.5 and a SPEED score of 16. Tear film stability is compromised (non-invasive break-up time: 3.3 s), and blink frequency is elevated (23.8 blinks/min). Objective findings include a thin lipid layer (Grade 1), reduced tear meniscus height (0.15 mm), and moderate meibomian gland dropout in the lower lid (Meiboscale Grade 2).
Beyond structural and functional imaging, novel molecular assays are emerging. These include measurements of interleukin-6, lactoferrin, and other tear proteomic signatures [11]. Artificial intelligence–driven composite indices, such as the Dry Eye Severity Index, which integrates TBUT, Schirmer, and meibography, show promise for personalized disease stratification. Though not yet routine, these tools point toward a future of precision medicine in which ocular biomarkers help inform the dosing or escalation of systemic biologics [12].
In practice, these diagnostics can be pragmatically integrated into a clinical workflow. First, symptom screening with DEQ-5 or OSDI, whether on paper or via an app, helps identify candidates for further testing. Tear osmolarity testing (≥ 308 mOsm/l) and MMP-9 detection (positive result or corneal staining) further refine the assessment of tear homeostasis and inflammation. Schirmer testing, with or without anesthesia, provides insight into aqueous production (< 5 mm in 5 min, suggesting a deficiency). Finally, automated imaging – capturing NIBUT, LLT, meibography, and tear meniscus height – allows disease subtyping, which is essential for identifying evaporative versus aqueous-deficient or mixed mechanisms.
These diagnostic advances carry multiple practical implications for autoimmune care. First, they enable earlier diagnosis, as objective testing has been shown to shorten the typical diagnostic delay associated with Sjögren’s disease [13]. Second, they promote treatment precision by allowing osmolarity-based treatment targets (< 308 mOsm/l), analogous to treat-to-target strategies in RA. Third, shared biomarkers such as tear MMP-9 may reflect systemic cytokine activity and offer an additional lens through which to monitor disease control. Fourth, preoperative ocular surface optimization, especially in patients undergoing cataract or refractive surgery while on biologics, is now supported by point-of-care tests that inform surgical risk stratification [8, 14].
Looking forward, artificial intelligence will likely enable automated detailed meibomian gland quantification and real-time prediction of inflammatory flares. Low-cost, handheld NIBUT devices will enter primary care, and multiplex tear microarrays may soon align with systemic panels already familiar to rheumatologists.
In conclusion, DED has evolved from a subjective complaint to a quantifiable inflammatory disorder with direct relevance to autoimmune medicine. Incorporating objective diagnostics such as LLT, NIBUT, and meibography into rheumatology workflows fosters earlier intervention, more personalized therapy, and improved ocular-systemic outcomes.