Introduction
Systemic lupus erythematosus (SLE) is a complex, chronic autoimmune disease characterized by widespread inflammation and damage in multiple organs, including the kidneys, skin, joints, cardiovascular system, and central nervous system [1]. The pathogenesis of SLE involves the inadequate clearance of cellular debris, leading to the abnormal activation of the immune system and the production of autoantibodies [2]. These autoantibodies form immune complexes that deposit in various tissues, causing inflammation and organ damage. A critical pathogenic pathway in SLE involves the activation of plasmacytoid dendritic cells, which secrete type I interferons – potent inflammatory molecules that exacerbate immune system dysregulation and contribute to disease progression [2].
Left untreated or inadequately managed, SLE can lead to severe organ dysfunction, irreversible damage, disability, and reduced quality of life. The burden of SLE on healthcare systems is significant, as the disease requires long-term treatment, careful monitoring, and multidisciplinary care and affects young people. In Poland, the lack of standardized, evidence-based clinical guidelines tailored to local healthcare resources and practices creates inconsistencies in diagnosis, treatment strategies, and patient outcomes [3].
The development of national guidelines for the management of SLE in Poland is essential to ensure evidence-based care by aligning clinical practice with international recommendations while adapting to local resources. These guidelines aim to optimize treatment strategies through clear recommendations for disease-modifying therapies, standardize patient management to reduce variability in care, and improve patient outcomes by focusing on early intervention, flare prevention, and minimizing organ damage. Additionally, the guidelines will guide healthcare policies by providing a foundation for assessing the cost-effectiveness and accessibility of therapies, ultimately enhancing patient care and reducing the socio-economic burden of SLE. In conclusion the purpose of these guidelines is to provide recommendations for the management of adult patients with SLE and to support their implementation within the Polish healthcare system. By implementing evidence-based recommendations, these guidelines will help clinicians make informed decisions, support patients in achieving better disease control, and contribute to a more efficient allocation of healthcare resources in Poland.
Methodology
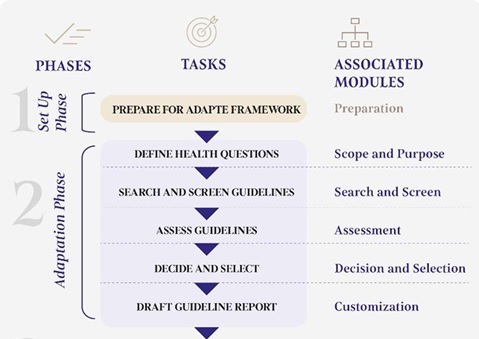
The adaptation process will be carried out in accordance with the ADAPTE Collaboration guidelines [4, 5] involving an interdisciplinary group of experts convened by the Guidelines International Network. The process will also follow the GRADE methodology [6, 7] and the ETD (Evidence to Decision) framework [8–10]. The formulation and finalization of statements will take place through a series of structured meetings. In the first step, the Working Group (WG) will create a list of clinical questions that the Polish recommendations will address (as illustrated in Figure 1). The established scope of clinical questions will be reviewed and approved by all members of the WG. Subsequently, existing guidelines regarding the management of patients with SLE will be systematically searched and evaluated based on predefined inclusion and exclusion criteria. All identified guidelines retrieved through the systematic search will be assessed for quality using the AGREE II instrument [5, 11]. The preparation and finalization of the guidelines will take place between November 2024 and September 2025.
Target groups or patient populations covered by the recommendations
Our guidelines address the principles of management for medical staff and other healthcare personnel involved in the treatment and care of adult patients with diagnosis of SLE. The scope of these recommendations explicitly excludes interventions related to the diagnosis of SLE, the pediatric population, and issues concerning comorbidities or non-pharmacological interventions.
Recipients of the guidelines
These clinical recommendations have been developed to support the treatment and management of patients with SLE, primarily by rheumatologists. The guidelines for the management of SLE are intended for a wide range of healthcare professionals and stakeholders involved in patient care to ensure effective and standardized management. However, the primary recipients are rheumatologists, who play a central role in diagnosing, treating, and monitoring patients with SLE. They are responsible for implementing evidence-based therapies and coordinating long-term care. Additionally, these guidelines are intended to assist physicians of other specialties, including primary care physicians, who serve as the first point of contact for many patients. Primary care physicians are essential for the early detection of SLE symptoms, prompt referral to rheumatologists, and the management of general health issues, comorbidities, and preventive care. Other specialists (e.g., nephrologists, cardiologists, dermatologists, neurologists) are also included as recipients of these guidelines because SLE is a multi-organ disease that requires a multidisciplinary approach to care. Finally, healthcare administrators and policymakers are addressed as recipients, as they are essential for supporting the integration of these guidelines into the healthcare system by a) allocating resources for optimal SLE management, including access to therapies, b) ensuring multidisciplinary care pathways and collaboration among healthcare units, and c) developing strategies to improve patient access to specialized care and treatment options.
The recommendations emphasize the importance of collaboration with rheumatologists to ensure comprehensive and coordinated care within the Polish healthcare system.
Criteria for considering guidelines
We will include all clinical guidelines published between 2019 and 2024 that focus on the pharmacological management of SLE. The research strategy will involve a combination of search terms such as “systemic lupus erythematosus” and “clinical recommendation” or “practical statements (guidance)”. Guidelines will be included if they are available as full-text publications on the Polish Society for Rheumatology (Polskie Towarzystwo Reumatologiczne – PTR) website. A language restriction will apply to English, Spanish, German, and Polish. The diagnosis of SLE in the guidelines must be established according to either the American College of Rheumatology (ACR) classification criteria or the Systemic Lupus International Collaborating Clinics (SLICC) classification criteria [12, 13].
Search methods for identification of studies
The clinical questions were formulated using the PICO format (Patients, Intervention, Comparator, Outcome). The target population will include adult patients with SLE and pregnant women, with the involvement of specialists such as nephrologists, dermatologists, neurologists, immunologist and other relevant experts within the WG.
We will conduct a comprehensive search in the following sources:
databases: GIN (Guidelines International Network), PubMed, and Embase;
international rheumatology societies: ACR, The European Alliance of Associations for Rheumatology (EULAR), World Health Organization (WHO);
national rheumatology societies: societies from Europe (i.e. British Society for Rheumatology – BSR, German Society for Rheumatology, Italian Society for Rheumatology, Spanish Society of Rheumatology), North America (Canadian Rheumatology Association), and Australia.
The search process will be finalized by November 2025.
Scope of interventions included in the guidelines
The guidelines primarily address issues related to the treatment of patients with SLE through pharmacological interventions, along with aspects of care organization. Table I presents aspects included to recommendations.
Table I
Scope of interventions included in the guidelines
The WG comprises individuals with extensive specialized knowledge, including: 1) experienced rheumatology specialists (with over 10 years of experience; up to 12 members); 2) experts in fields treating organs specific manifestations; 3) a patient representative. The group is balanced in terms of sex to ensure diversity. The chairperson of the WG collected conflict of interest (COI) declarations from all members and assessed them for potential conflicts. In cases where a conflict of interest was identified, appropriate actions were taken in accordance with WHO recommendations for guideline development and conflict-of-interest management.
The WG will develop overarching principles and specific recommendations regarding the management of SLE. The overarching principles will provide a conceptual framework for patient care, emphasizing key aspects such as patient-centered care, early diagnosis, multidisciplinary management, treatment optimization, and long-term monitoring to improve outcomes and quality of life for patients with SLE. The WG will also work on specific recommendations concerning the use of pharmacological treatments, including hydroxychloroquine, glucocorticosteroids, immunosuppressive drugs (methotrexate, mycophenolate mofetil, azathioprine, cyclophosphamide), calcineurin inhibitors: cyclosporine, tacrolimus, voclosporin, and biologic therapies: belimumab, anifrolumab, rituximab. In addition to pharmacological treatment, the recommendations will provide advice on: 1) treatment strategies and therapeutic targets; 2) assessment of treatment response; 3) combination and sequential therapies; 4) tapering of therapy and special situations like pregnancy.
The work will be based on a consensus-driven approach among panel members for both the overarching principles and specific recommendations. In cases where consensus cannot be reached, approval of a recommendation or position will require a majority vote of ≥ 80% of the WG members.
Recommendation formulations
In formulating the recommendations for the Polish guidelines, the experts will analyze existing source recommendations for their applicability in the national context. The EULAR and ACR guidelines for the management of patients with SLE will serve as the primary international references. All relevant guidelines identified during the systematic search will be assessed using the AGREE II instrument, with particular emphasis on the methodology domain to ensure credibility.
The formulation of recommendations will also consider the following three key questions from the ETD framework:
What will be the impact of the intervention on health equity?
Is the intervention accepted by patients and those delivering it?
Is the implementation of the intervention feasible?
After selecting the recommendations, the wording of the proposals will be discussed in detail. Any contentious issues will be resolved through discussion and consensus-building among the members of the WG. In situations where direct scientific evidence is lacking, the WG will formulate Good Practice Guidance (expert panel statements rather than formal recommendations). These statements will be developed when the panel determines that: 1) the benefits of the intervention are substantial; 2) adverse effects are minimal; 3) patient values and preferences are clear; 4) the intervention is cost-effective; 5) the intervention is clearly acceptable, or 6) the intervention promotes social justice or equality of access. All experts in the WG will be required to approve the final version of the document, which is scheduled for completion in November 2025.
Implementation plan
After a thorough review of the recommendations by two independent external reviewers, the final document will be published in both Polish and English versions. The Polish version will be available on the PTR website, while the full-text English version will be published in the scientific journal Reumatologia, in both electronic and print formats. All versions will be made available as open-access publications.
The implementation strategy for these guidelines will include a series of informational and educational activities aimed at physicians to promote the adoption of the recommendations. The strategy will involve: 1) dissemination of knowledge through publication in scientific journals; 2) presentations at national and international scientific conferences; and 3) distribution of the electronic version of the guidelines during the period 2025–2026.
These initiatives are intended to ensure broad accessibility and awareness of the guidelines among healthcare providers, facilitating their integration into clinical practice.
Conclusions
These guidelines for the management of SLE in Poland will provide a comprehensive, evidence-based framework to support clinicians, healthcare providers, and policymakers in delivering standardized and effective care. By aligning clinical practices with international recommendations and adapting them to the specific needs and resources of the Polish healthcare system, these guidelines will optimize treatment strategies, improve patient outcomes, and reduce the socioeconomic burden of SLE.
The successful implementation of these recommendations will require collaboration among rheumatologists, other specialists, primary care physicians, and healthcare administrators to ensure a multidisciplinary and patient-centered approach. Continuous education, guidelines dissemination, and integration into clinical practice will be essential to achieve the intended benefits.
Ultimately, these guidelines will serve as a key step toward improving the quality of care for patients with SLE in Poland, enhancing their quality of life, and fostering a more efficient and equitable healthcare system.