Introduction
Osteoarthritis (OA) is a chronic degenerative joint disease which leads to increasing numbers of patients suffering from disability due to damaged joints such as the knee (with the highest frequency of occurrence), hip, hand and spine as a result of degradation of articular cartilage and inflammatory processes in the synovium and joint fat pad [1].
The number of patients struggling with OA has been increasing through the years. In 2020, 595 million people had OA, which is equal to 7.6% of the global population, and an increase of 132.2% in total cases since 1990 [2].
Even though research into the causes and pathomechanism of OA is still ongoing, it has already been discovered that there are several different factors as well as a complex pathomechanism leading to OA. The main factors responsible for development of OA include age, traumas of joints, diet and physical activity. The changes occurring in those areas have a significant impact on further pathological mechanisms leading to OA. There are studies suggesting that several changes start at the level of DNA, such as methylation or histone modification. The pathomechanism of OA can also be found in the synovial fluid due to the activation of its lymphatic system [3]. The activity of matrix metalloproteinases (MMPs), osteopontin, adipokines and pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α) or interleukin (IL)-1β, IL-1 and IL-6 also play a crucial role in the progression and severity of cartilage damage as well as in pain connected with this disorder [4].
Regarding the gut microbiota, it is responsible for harvesting energy through digesting food, immunological processes and protecting the organisms from pathogens as well as strengthening the permeability of the intestines. Changes in gut microbiota may affect the functions of host organisms, potentially increasing inflammation in the intestines and triggering release of pro- inflammatory cytokines, which may influence the development of OA.
The aim of this review is to summarize the factors contributing to OA and how their modifications can influence changes in the gut microbiome (GMB) composition in order to decrease inflammatory processes leading to OA.
Factors contributing to osteoarthritis
There are several different factors which can lead to development of OA. One of them is mechanical stress, which can be determined by age, traumas, weight overload caused by diet, lack of physical activity and changes in metabolism. It has been shown that the force which exerts pressure on the knee is 3 to 6 times one’s body weight. So, the more the patient’s weight increases, the greater is the pressure applied to the knee, leading to mechanical stress and cartilage degeneration. Furthermore, there is evidence that reducing body weight has a beneficial impact on the knee, leading to decreased risk of development of OA in the knee joints [5, 6]. To provide a proper equilibrium between anabolic and catabolic processes, the chondrocytes should be under a certain pressure, due to which homeostasis of the pro-inflammatory and anti-inflammatory processes is not disturbed [7]. Even though physical activity can have a beneficial effect on the symptoms of OA, it was found that female athletes with a history of bearing weights had a 2–3-fold increased risk of OA [8]. Moreover, another study found that heavy lifting or spending the majority of work standing was associated with hip OA [9]. It is established that mechanical stress leads to increase of the pro-inflammatory cytokines, prostaglandin E2 and nitric oxide [10]. The injury caused mechanically can lead to disruption of the equilibrium between superoxide and superoxide dismutase 2, which leads to degeneration of the cartilage as well [11, 12].
Furthermore, obesity has not only been one of the most common causes of OA but also the main factor of changing GMB composition as well. In experiments on mice it was observed that obesity was correlated with abundance of bacteria – Firmicutes and Bacteroides. It has been demonstrated that the largest population of people who suffer from OA have higher abundance of Lactobacillales, particularly Streptococcus spp. [13]. Moreover, it has been shown that the abundance of Firmicutes – which is estimated at around 20% of the gut microbiota – is correlated positively with higher pain score as well as lower functionality [13].
Not only is obesity closely connected with mechanical overload, which leads to cartilage degradation, but also through the increased presence of adipokines it affects and disrupts the articulation. Adipokines such as leptin, adiponectin and lipocalin 2 produce pro- inflammatory cytokines such as IL-6 and TNF-α, leading to remodeling of subchondral bone and degradation of extracellular matrix (ECM) of the cartilage [14–16]. Furthermore, there has been a proven correlation between high-fat diet and osteocyte changes [17].
Impact of gut microbiome on development of osteoarthritis
There are several pathways through which GMB can affect OA. One of them is through the activation of macrophages, their migration and their pro-inflammatory effect. They may be affected by the intestine-blood barrier, which further leads to cartilage damage [18]. Wu et al. [1] stated that cartilage degeneration is caused by several processes including the secretion of pro-inflammatory cytokines such as IL-1, IL-6, IL-8 from the pro-inflammatory M1-like macrophages activated by the T-helper 1 cells located in the synovial lining layer as well as the MMPs. Not only M1-like macrophages are located there but also the anti-inflammatory M2-like macrophages – activated by T-helper 2 cells – which secrete e.g. IL-4 and IL-10. However, the ratio between the abundance and activation of M1 and M2-like macrophages is in favor of the pro- inflammatory ones, which as a result of this disproportion leads to degeneration of cartilage [1].
Another way in which GMB can affect OA is through peptidoglycan, which is responsible for expression of MMPs and also for pro-inflammatory cytokines. The receptors which are involved in such expression include Toll-like receptor 2 and NOD-like receptor 1, influencing systemic innate immunity. Furthermore, lipopolysaccharide (LPS) can affect inflammation in adipose tissue, leading to activation of cytokines, adipokines and growth factors. Moreover, the LPS forms complexes which have catabolic and damaging impacts on joints by e.g. increasing nuclear factor κB (NFκB) levels, upregulating levels of pro-inflammatory cytokines and receptor activator of NFκB ligand, and increasing synthesis of MMPs. Moreover, LPS is involved in OA by activating the complement pathway in chondrocytes [18]. Chondrocytes in OA cartilage can express pro-inflammatory cytokines such as IL-1. The MMP expression is highly controlled by them and can influence ECM remodeling, with its further consequences [19].
Causes of dysbiosis of gut microbiota
Causes of dysbiosis include infections, inflammation, intake of drugs, diet, and physical activity [20, 21].
There are three types of dysbiosis [22]:
type I – caused by the loss of beneficial organisms,
type II – involving excessive growth of harmful organisms,
type III – loss of diverse GMB.
Impact of early stages of life on gut microbiota composition
There are reasons to believe that the composition of the GMB during early stages of life may be one of the causes of obesity, which is one of the main factors influencing development of OA [23]. It is known that the microbial composition of the gut of a newborn can be influenced by types of bacteria in the birth canal during vaginal delivery, ways of feeding the newborn, the surrounding and environment of the baby (including types of animals living with the baby or close to the baby), and medications (including antibiotics) [24–26]. After the first 3 years of living, the gut microbiota forms itself into a unique, individual component, which can be considered as a “set of fingerprints” [27–29]. Even though the composition of the gut microbiota is an individual “fingerprint”, 90% of the GMB is mostly composed of phyla such as Bacteroidota (Bacteroides and Prevotella) and Firmicutes(Lactobacillus, Bacillus, Clostridioides, Enterococcus, Staphylococcus). The abovementioned phyla are responsible for keeping the relationship between GMB and host at an optimal level, especially as regards metabolic processes, energy from them and immunological barriers [27–30].
Symptomatic slow-acting drugs for osteoarthritis
Even though there is still research being conducted, there are findings suggesting that symptomatic slow-acting drugs for osteoarthritis (SYSADOA) may play an important role in treating OA. The drugs present chondroprotective properties which may help in pain alleviation, or delay or stabilize the pathomechanism of OA. The research of Permuy et al. [31] showed that among all the SYSADOA the highest effectiveness was demonstrated by diacerein and risedronate, which increase bone density and have a beneficial influence on the cartilage surface. The results gained through application of hyaluronic acid show that it improves cartilage swelling, but it does not affect the synovium in any visible way. Chondroitin (CS) and glucosamine sulfate had no significant effect on the structure of cartilages, synovial membranes or subchondral bone.
Furthermore, the Glucosamine/Chondroitin Arthritis Intervention Trial(GAIT) found that the combination of CS and glucosamine had a significant effect on reducing pain and increasing the functionality of patients with OA suffering from moderate or severe pain levels [32].
According to the review of Shmagel et al. [33], CS oral supplementation may increase the abundance of Bacteroides in GMB. There is some evidence that Bacteroides has anti-inflammatory properties and can take part in cancer immunotherapy and prevention. Even though it lacks strong evidence in the literature, there are reasons to believe that anti-inflammatory properties of bactericides and their modulations may be among the potential areas of research and further development [34].
Role of physical activity
One of the factors having a huge impact on the GMB as well as on OA is physical activity. It has been firmly established that physical activity has an impact on several metabolic factors, including hyperglycemia, obesity and hyperlipidemia. Furthermore, it not only can reduce body mass, delay onset or diminish the consequences of OA but can also change the GMB in favor of more beneficial phyla and increase the diversity of bacteria. Physically active people tend to exhibit greater richness of the GMB in comparison to those with a sedentary lifestyle. Not only lifestyle has an impact on the richness of the GMB but also ways of approach to sport – amateur or professional – as well as sex of the person; e.g. in active women there is a higher abundance of health- promoting bacteria such as Faecalibacterium prausnitzii, Roseburia hominis and Akkermansia muciniphila [35].
Physical activity not only affects the composition of the GMB but also has a crucial role in primary and secondary prevention of joint injuries. As a result of regular physical activity, the flexibility, strength and balance of a human being are improved [36]. Furthermore, exercises help in controlling weight due to the fact that obesity is one of the risk factors for developing OA. Excessive body weight causes stress on the knees, hips and lower back, which hold the weight of a human body [37]. As a result of regular exercises, especially those improving strength, there is lower risk of muscle atrophy, which may lead to falls, resulting in serious injuries of the joints and their cartilages. Moreover, exercises for elderly people are also highly recommended even though their intensity or duration might not be the same as for the younger population [36].
Exercises for flexibility lengthen the muscles, which results in increased range of motion of the joints. Due to the fact that the hip and knee joints hold most of the human body’s weight, those are the area on which the exercises should focus. For older people the exercises should be done at least twice a week and they should include inner leg stretches, hip and lower back stretches, as well as double hip rotation. Furthermore, strength training should also be included, because it prevents the reduction of muscle mass. Resistance training can include additional tools such as resistance bands or ankle/ hand weights, but body weight alone may be sufficient to increase muscle mass and strengthen the muscle. The patients should not overexercise; 2 training sessions per week should suffice [36]. Research shows that the main role in strengthening the knee joint is to strengthen the muscles which run along the frontal part of the knee – mainly the quadriceps. An exercise which can help in strengthening the quadriceps is “wall squads”, repeating them around 8 to 10 times. It is important for the patient not to bend the knees more than 90 degrees [37]. The most recommended aerobic exercises are those that avoid putting too much strain on the joints, such as swimming or pool exercises. This especially applies to elderly or obese people [36].
It is important to add that to prevent injuries, it is highly recommended for people to wear proper shoes during their training, to do warm-ups beforehand, and to cool down at the end. Moreover, flat surfaces are recommended, to reduce the risk of a twisted knee or other injury [37].
Role of diet
Even though further studies are needed, there are reasons to believe that the Mediterranean diet with fruits and herbs can be beneficial for patients struggling with OA due to its anti-inflammatory effects and beneficial effect on weight control. The results of the diet include decreased symptoms, reduced pain, increased mobility and reduced cartilage degeneration [38].
There is not enough evidence in the literature, but there are suggestions that increased consumption of certain food products with antioxidative properties such as strawberries or ginger (which contains gingerol and shogaol) help reduce inflammation [38].
According to the Association of UK Dietitians, patients struggling with OA should consume the long-chain omega-3 polyunsaturated fatty acids which occur in oily fish; it is recommended to consume two portions of it weekly. If it is impossible to consume enough fish, it is recommended to consume 450 mg of eicosatetraenoic acid and docosahexaenoic acid daily. Due to its anti-inflammatory properties, long-chain omega-3 polyunsaturated fatty acids are highly recommended in the patients’ diets. On the other hand, omega-6 polyunsaturated fats should be removed from the diet and replaced with mono-unsaturated fats such as olive oil [39]. Even though there is not enough evidence in the dietary area, people suffering from OA are recommended to consume an adequate daily intake of vitamins, minerals and unsaturated fatty acids in a healthy, balanced diet.
Impact of excess calories and food quality on gut microbiome
It has been reported that the caloric load was the most important factor influencing the occurrence of these correlations. However, the caloric load is not the only factor affecting GMB. While higher than the required number of calories per day can be a reason for further negative consequences such as obesity, in the case of rugby athletes consuming around 4,500 kcal per day, their GMB diversity is high. Furthermore, the fact that the quality of consumed food is also an important part of the diet, not only its quantity, shows that eating specific types of consumed products can reduce inflammatory processes [40]. There are more and more studies showing that anti-inflammatory products such as polyunsaturated fats can be beneficial in decreasing the low-grade inflammation and its symptoms in OA such as pain and the patient’s decreased functionality.
Role of probiotics and supplements
Even though there is not strong supporting evidence in this area due to the lack of studies and difficulties in conducting them, there are also some data showing that obtaining – preferably in dietary products instead of supplements – adequate amounts of vitamins such as A, C, E, D, and K may be beneficial for those with OA. There are studies suggesting that vitamin D is not only responsible for mineral homeostasis and bone metabolism but also to some extent for muscle strength. In the case of vitamin K, there are reasons to believe that its insufficiency may have consequences for the OA pathomechanism. Mainly it may be caused by the functionality of vitamin-K-dependent proteins which are found in bones and cartilage [41].
The majority of patients affected by OA are over 60 years old. It has been proven that especially older patients suffer from nutritional deficiencies due to decreased appetite or limited ability to take care of themselves. Research on irritable bowel disease and colitis showed that vitamin D supplementation may be beneficial for increasing epithelial cell resistance and suppression of inflammatory responses [42]. It has been shown that increased abundance of vitamin D may influence changes in GMB such as increased concentrations of Clostridioides and Bacteroides, and decreases of Lactobacillaceae and Firmicutes phylum [42]. Moreover, Lactobacillus spp. may be responsible for anti-inflammatory and anti-tumorigenic effects due to production of beneficial metabolites. It was reported that the disease severity may have been lower in comparison to the control, placebo group [43].
Supplementing the most crucial vitamins, elements as well as fiber may help with correcting their deficiencies, leading to positive changes in the composition of the GMB, resulting in decreasing low-grade inflammation affecting patients’ joints. The decreased abundance of butyrate- producing bacteria may be caused by decreased intake of fiber. It has been suggested that bacteria which produce those metabolites may be one of the factors responsible for anti-tumorigenic effects such as induction of apoptosis, inhibition of proliferation of neoplastic cells and restricting tumor angiogenesis [44]. To increase their concentration the intake of fiber needs to be increased, which results in increasing abundance of butyrate-producing phyla such as Bifidobacteria and Roseburia [44].
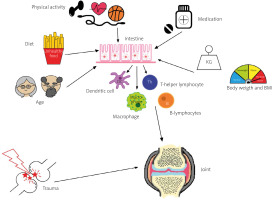
Factors directly or indirectly influencing the composition of the GMB and its impact on joints are shown in Figure 1.
Conclusions
Even though there are not enough long-term data, there is an evidential basis to support further research into correlations of body mass, diet, physical activity, drugs and supplementations with composition of the GMB and its further impact on OA.
Further investigations may be beneficial to find out more about the impact of early-stage GMB on OA development in later stages of life. There are certain indications showing that diets favorable for the GMB and with anti-inflammatory properties can reduce low-grade inflammation, decreasing degenerative processes occurring in the joints.
There is a lack of strong and direct recommendations e.g. about the frequency of regular physical activity or which meals are the most beneficial for the patients’ unique GMB. However, there are reasons to believe that the Mediterranean diet with antioxidants and vitamins as well as physical activity focusing on flexibility, increasing muscle mass and aerobic exercises may be used as methods of primary or secondary prevention in OA development.
The impact of Lactobacillus spp. on formation of the GMB – in the early stage of life – may be a potential area of development, especially as Lactobacillus spp. play several roles in the human body and may be supplemented by patients. Those values bring new possibilities for further research and discoveries in modifications of the GMB.