Introduction
Rheumatoid arthritis (RA) is the most common form of chronic inflammatory autoimmune arthritis that affects ~1.5% of the general population worldwide [1]. Persistent synovitis is the hallmark of RA and is associated with continuous elevated concentrations of proinflammatory cytokines.
Uncontrolled active RA commonly leads to severe articular damage, and numerous comorbidities, including cardiovascular diseases and osteoporosis leading to significant patient disability and physical impairment [2].
Serum 14-3-3η proteins belongs to a widespread family of eukaryotic cells proteins. These highly conserved proteins form homo and heterodimers between isoforms. The characteristic amphipathic binding groove-like structure of the dimeric molecule [3] allows the binding of 14-3-3η proteins with over 200 signaling molecules, such as transmembrane receptors, kinases and phosphatases [4]. The seven human 14-3-3η protein isoforms were found to be involved in diverse biological activities, such as regulation of cellular proliferation and signal transduction [5].
Rheumatoid arthritis patients had a higher serum 14-3-3η level than matched controls [6], and it may therefore be a potential helpful biomarker to diagnose of RA alongside the classical anti-citrullinated protein antibodies (ACPA) and rheumatoid factor (RF) [7].
At the European Alliance of Associations for Rheumatology (EULAR) congress 2014, it had been suggested that the diagnostic certainty of RA can be improved by assessing 14-3-3η protein and may cover the shortages of RF and ACPA in detection of RA patients [8].
However, the findings regarding the relationship of 14-3-3η protein with systemic inflammatory markers and disease activity in RA were conflicting [9, 10], and the association between the 14-3-3η protein and disease activity in RA remains to be clarified.
In contrast, local and systemic bone loss are hallmarks of RA. The prevalence of osteoporosis in RA is ~30% and may reach 50% among post-menopausal females, which is higher (~2-fold) than the prevalence reported in the general population in the same age group [11, 12] and associated with elevated risk of fracture [13].
A meta-analysis study showed that the fragility fractures incidence in RA is more than double that of the general population (33 and 15/1000 person-years, respectively) [14]. Factors associated with increased risk of osteoporosis in RA patients include the use of steroids, reduced physical mobility, older age, low body mass and female gender [15]. However, the development of osteoporosis in RA can also be attributed to other different mechanisms, and thus may a warrant different treatment approach [2].
It had been observed that activated 14-3-3η protein can induce matrix metalloproteinase (MMP)-1 and MMP-9 and receptor activator of nuclear factor κB ligand (RANKL) [10] and thus may contribute to joint damage [16]. Given the strong association of the 14-3-3η protein with MMP_1 and MMP_3 in the serum and synovial fluid [6], it can be hypothesized that 14-3-3η protein may impact the course of bone remodeling and may promote the development and progression of osteoporosis.
This study aimed to explore the potential association of serum 14-3-3η protein level with disease activity and osteoporosis in Egyptian patients with RA.
Material and methods
Participants
A total of 200 RA cases were enrolled in the current study. The 2010 EULAR and American College of Rheumatology (ACR) classification criteria for RA were used to identify RA patients [17]. Patients were collected from the outpatient clinic of the Rheumatology and Rehabilitation Department, Mansoura University Hospital from November 2020 to May 2021. Also, 200 age and gender-matched healthy subjects were enrolled in this study.
Exclusion criteria
Exclusion criteria included postmenopausal females, patients with other rheumatic autoimmune diseases, severe infection, diabetes mellitus, hypertension, liver or kidney disease, malignancies, endocrinal diseases, and pregnant or lactating females. Patients with current intake of glucocorticosteroids, anticonvulsants, estrogen or anti-coagulant, alcohol abusers, or other factors that are associated with secondary osteoporosis were also excluded from the study [18].
The aim and procedures of the study were discussed thoroughly with all participants. Prior to inclusion in the study, the participants provided written consent for participation. Eleven participants (7 patients and 4 controls) did not provide written consent, and an additional 5 patients and 2 controls were excluded due to meeting the exclusion criteria, leaving 188 RA patients and 192 controls eligible for participation in the study.
Data collection
Each participant underwent detailed medical history taking as well as clinical examination. Medical data of all patients were analyzed. Data collection included age, gender, past medical history, current and past drug intake. In rheumatoid arthritis patients, the disease duration, duration of morning stiffness, tender joint count (TJC) and swollen joint count (SJC) were recorded.
The activity of RA was measured by disease activity score 28 (DAS28) based on the erythrocyte sedimentation rate (ESR) (DAS28–ESR) [19]. The intensity of perceived pain was evaluated using the 100-mm Visual Analogue Scale (VAS) [20].
Measurement of bone mineral density
Dual energy X-ray absorptiometry (DEXA) at the proximal femur was used to measure bone mineral density (BMD) in all participants who were categorized into normal, osteopenic, or osteoporotic according to the definition of the World Health Organization (WHO) of the recorded T score [21].
Laboratory assessment
Samples of venous blood were withdrawn from all participants in the morning after an overnight fasting. Laboratory investigations included measurement of ESR, and of C-reactive protein (CRP) levels by the immune-nephelometric technique (kits from Tianjin Biotechnology Co, Ltd) that measures the agglutination of particles by quantifying the scattered light.
Rheumatoid factor (RF) levels were measured by the nephelometry method (kits from Tianjin Biotechnology Co, Ltd) following the manufacturer’s instructions and RF above 20 IU/ml is not considered positive. Enzyme-linked immunosorbent assay (ELISA) was utilized for determination of anti-cyclic citrullinated peptide antibodies (anti-CCP) according to the manufacturer’s instructions (Toscana biomarkers, Siena, Italy kits).
At 20 U/ml and higher, an anti-CCP test is considered positive. A commercially available human IL-6 ELISA kit (GenProbe, Diaclone, France) was used to measure IL-6 serum level following the instructions of the manufacturer.
Serum 14-3-3η levels were evaluated by the quantitative 14-3-3η ELISA (Augurex Life Sciences Corp, Vancouver Canada) according to the manufacturer’s protocol. The positivity of the serum 14-3-3η was defined as ≥ 0.19 ng/ml [16].
Statistical analysis
The SPSS Statistics was used to edit, code, summarize, and upload the acquired data (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.).
Information was supplied, and appropriate analysis was performed for each parameter based on the type of data gathered. A regression analysis model was implemented to identify which variables may impact BMD in RA cases. The level of p < 0.05 was set as the threshold of significance.
Bioethical standards
All procedures were performed according to the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. IRB Proposal Code: R.21.11.1528 – 2021/11/21. Clinicaltrials.gov registration date: 04/05/2022, code: NCT05366608. All study participants were given the opportunity to give their informed consent.
Results
Rheumatoid arthritis patients vs. controls
This study enrolled 188 RA cases, 159 (84.6%) females and 29 (15.4%) males, with a mean age of 38.6 ±7.1 years (range, 27–50 years). The study also included 192 controls, 160 (83.3%) females and 32 (16.7%) males with a mean age of 39.3 ±6.3 years (range from 28 to 49 years).
The rheumatoid arthritis group had a significantly higher ESR level than the control group (64.2 ±31.5 and 26.3 ±7.1 respectively, p < 0.001) and a significantly higher CRP level (40.6 ±18.8 and 3.2 ±1.1 respectively, p < 0.001). The average BMI of the RA group and control group was 22.1 ±1.3 and 22.2 ±1.3 kg/m2 respectively. Patients and control subjects were matched for age, gender and BMI.
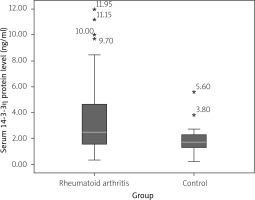
The median serum concentrations of 14-3-3η protein in the RA group and control group were 2.49 ng/ml (IQR = 3.10) and 1.71 (IQR = 1.02) respectively (Fig. 1). This difference was significant (p < 0.001).
Comparison of the rheumatoid arthritis-related features among the rheumatoid arthritis groups
Based on the WHO definition of the BMD categories, the RA patients were categorized into the RA-control group (n = 84), the RA-osteopenia group (n = 59) and RA-osteoporosis group (n = 45). Table I compares the age, gender and the clinical and laboratory features among the RA-control, RA-osteopenia and RA-osteoporosis groups. Age, gender, duration of RA and the medications used showed no significant difference among the groups.
Table I
General characteristics of the rheumatoid arthritis group and control group
[i] ACPA – anti-cyclic citrullinated peptide antibodies, CRP – C-reactive protein, DAS28–ESR – disease activity score 28 based on the erythrocyte sedimentation rate level, HCQ – hydroxychloroquine, LF – lactoferrin, MTX – methotrexate, RF – rheumatoid factor, SJC – swollen joint count, TJC – tender joint count.
However, the duration of morning stiffness, the TJC and SJC differed significantly among the groups being highest in the RA-osteoporosis group and lowest in the RA-control group (p = 0.027, p = 0.033 and p = 0.016 respectively).
Similarly, the ESR, CRP serum level and IL-6 serum level were also significantly different between study groups, being highest in the RA-osteoporosis group followed by the RA-osteopenia group and lowest in the RA-control group (p = 0.038, p = 0.003 and p = 0.026 respectively).
As shown in Table I, the mean DAS28-ESR in the RA-osteoporosis, RA-osteopenia and RA-control was 4.5 ±0.9, 4.2 ±0.8 and 3.9 ±0.9 respectively. These differences of DAS28–ESR among the groups were significant (p < 0.001).
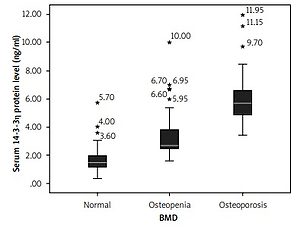
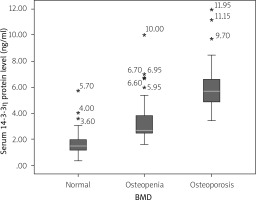
Comparison of serum level of 14-3-3η protein among rheumatoid arthritis groups
Figure 2 compares the 14-3-3η protein serum level among the RA-control, RA-osteopenia, and RA-osteoporosis groups. A significantly higher median serum level of 14-3-3η protein was found in the RA-osteoporosis group (median = 5.68, IQR = 1.77) than RA-osteopenia group (median = 2.69, IQR = 1.36) (p < 0.001), and RA-control group (median = 1.47, IQR = 0.86) (p < 0.001). Similarly, the difference of the serum 14-3-3η protein between the RA-osteopenia group and RA-control group was significant (p = 0.003).
Linear regression analysis to explore predictors of bone mineral density in rheumatoid arthritis patients
Linear regression analysis was performed to determine the variables strongly associated with BMD among the RA patients. The linear regression analysis revealed that BMD in RA patients was associated with serum level of 14-3-3η protein (p = 0.006), IL-6 (p = 0.044) and DAS28-ESR (p = 0.025) (Table II).
Table II
Linear regression analysis to explore rheumatoid arthritis-related features associated with bone mineral density
Correlation between serum 14-3-3η protein level and rheumatoid arthritis-related features
Serum level of 14-3-3η protein showed a significant association with the clinical and laboratory determinants of the disease activity. In addition, serum level of 14-3-3η protein showed significant correlation with DAS28–ESR (p = 0.003). Regarding the pro-inflammatory cytokines, serum level of 14-3-3η protein was significantly correlated with IL–6 serum level (p = 0.025) (Table III).
Table III
Correlation between serum 14-3-3η protein level and age of the patients and rheumatoid arthritis-related features
However, as demonstrated in Table III, serum 14-3-3η protein level showed no significant correlation with age of RA patients, RA duration, RF titer or anti-CCP titer.
Association between serum level of 14-3-3η protein and gender and drugs used
As shown in Table IV, there was no significant difference in serum level of 14-3-3η protein between RA female and male patients. Similarly, serum level of 14-3-3η protein did not show significant difference between RA patients taking corticosteroids (current or previous), methotrexate (MTX), lactoferrin (LF), hydroxychloroquine (HCQ) or biological treatment compared to those not taking these drugs.
Table IV
Association between serum 14-3-3η protein level and gender and drug used in rheumatoid arthritis patients
Discussion
The major findings of the current study were:
serum 14-3-3η protein level was significantly higher in RA patients than controls,
serum level of 14-3-3η protein was significantly correlated with the clinical and laboratory determinants of disease activity,
the RA-osteoporosis group had significantly higher serum 14-3-3η protein than the RA-osteopenia group and RA-control group.
Similarly, the difference of the serum 14-3-3η protein level between the RA-osteopenia group and to RA-control group was significant.
Several previous studies have assessed the serum 14-3-3η protein level in RA cases and its association with disease activity, but provided conflicting results. In the present study it was found that serum level of 14-3-3η protein was significantly increased in RA patients and serum 14-3-3η protein level was closely associated with activity parameters.
Our findings were consistent with those of Maksymowych et al. [10] who found that serum 14-3-3η protein level in healthy subjects was significantly lower than in patients with early RA and established RA and the serum 14-3-3η protein level was significantly correlated with the ESR and CRP levels.
Gong et al. [22] observed that RA patients had a significantly higher serum level of 14-3-3η protein than the matched controls, and 14-3-3η protein was significantly correlated with the disease activity as well as the proinflammatory cytokines in the RA patients.
The study of Guan et al. [23] revealed that the median serum level of 14-3-3η protein in RA patients was significantly higher than that in controls and was significantly correlated with CRP, ESR, and DAS28 in RA patients.
In Japanese patients with established RA, patients with positive 14-3-3η had a significantly higher DAS28 score, ESR level and CRP level than patients with negative 14-3-3η [9]. Tu et al. [24] found that RA patients had a significantly higher serum 14-3-3η protein level than in osteoarthritic patients and control individuals.
The 14-3-3η expression in serum showed a direct correlation with duration of RA, ESR and DAS28 score. That study concluded that serum 14-3-3η level seems to be a promising candidate for prediction of the risk of RA and can reflect disease activity. Such findings are consistent with the results of our study.
It has been found that serum 14-3-3η protein level enhances various pro-inflammatory molecules and therefore may play a significant role on the pathogenesis of RA [25]. Our results showed that serum level of 14-3-3η protein is significantly correlated with clinical and laboratory indices of disease activity, DAS28–ESR and with IL-6 in RA patients.
On the other hand, Dammona et al. [26] found that although serum 14-3-3η protein level was significantly higher in RA than controls and serum 14-3-3η protein was significantly correlated with serum RF and anti-CCP, the protein did not show significant correlations parameters of disease activity including ESR, CRP, DAS28 or musculoskeletal ultrasound parameters. The discrepancy between the findings of that study and ours can be attributed to the lower sample size and type of RA patients enrolled in that study (that study enrolled 30 patients with early RA).
Osteoporosis is at least twice as frequent in RA patients than matched controls [11, 12] adding to the disability burden of RA [27]. Thus, better understanding of the pathophysiological mechanisms underlying development of osteoporosis in RA is crucial.
In the present study, a significant difference in the serum levels of 14-3-3η and IL–6 among RA-control, RA-osteopenia, and RA-osteoporosis groups was found being lowest in the RA-control group and highest in the RA-osteoporosis group. These findings were in agreement with the findings of a previous studies [22, 28], and showed an inverse correlation with lumbar and femoral BMD among RA patients [22].
Zeng et al. [29] found that serum 14-3-3η protein was significantly higher among RA cases than controls and the regression analysis identified 14-3-3η as an independent risk factor for RA-related osteoporosis. Such findings agree with the results of the present study. These findings suggest that 14-3-3η can be an early predictor for development of osteoporosis in RA patients.
In confirmation of the association between serum 14-3-3η protein level and osteoporosis, a previous study reported that 14-3-3η serum level was significantly correlated with C-terminal telopeptide of type I collagen (I-CTX) and inversely correlated with N-terminal propeptide of type I procollagen (PINP) [28]. Both CTX-I and PINP are well-recognized biomarkers for bone remodeling [30].
In chronic inflammation, IL-6 upregulates the serum receptor activator of nuclear factor κB ligand (RANKL), resulting in osteoporosis [31, 32]. The extracellular 14-3-3η protein can induce the proinflammatory mediators that are directly involved in cartilage and bone degradation [33, 34]. Serum IL-6 level is significantly associated with osteoporosis in RA patients. In addition, IL-6 showed a significant correlation with 14-3-3η [28].
Consequently, the study concluded that serum level of 14-3-3η protein influenced bone remodeling and contributed to progression of osteoporosis. In rheumatoid arthritis, the severity of osteoporosis was correlated with the degree of inflammation in terms of elevation of proinflammatory mediators including CRP, TNF-α and IL-6 [35]. These findings were consistent with the findings of the present study.
Study limitations
The present study had its limitations. First, RA patients with concomitant intake of glucocorticoids, and other medications that may cause osteoporosis were excluded. However, in clinical practice several RA patients may take such medications. If such patients were included, the current results can be affected. Second, the radiological data regarding joint damage and progression that may add to the value of assessment of 14-3-3η levels were not assessed in the present study.
A future long-term study with a more comprehensive RA population is necessary for confirmation of the findings of the present study and to evaluate the prognostic value of this biomarker. An area of future study is to explore the effect of targeting the serum 14-3-3η protein on progression of osteoporosis.
Conclusions
Serum 14-3-3η protein is significantly elevated in RA patients in comparison with control subjects and is significantly correlated with parameters of disease activity. The RA-osteoporosis group had significantly higher serum 14-3-3η protein than the RA-osteopenia group and RA-control group. Serum 14-3-3η protein can be a promising marker that reflect RA activity and predict presence of osteoporosis in RA patients.