Introduction
Systemic lupus erythematosus (SLE) is a very heterogeneous and complex autoimmunological disease sometimes difficult to recognize and treat. Tremendous diagnostic progress, wider awareness of consequences of damage, and new therapy development have significantly reduced mortality and improved prognosis. The risk factors for organ damage progression and factors of poor prognosis force us to use the most effective possible treatment.
Our clinical experience gives us the basis for more aggressive therapy when we are dealing with a severe course of the disease, lupus nephritis, or symptoms of organ damage already at the beginning of the lupus diagnosis. Irreversible organs damage is a cause of permanent reduction in quality of life and disability of patients with SLE. We have evidence that one organ failure leads to another, regardless of disease activity control and treatment [1].
For more effective management, limited complications, and less progression of secondary organ damage we should identify the goal of the treatment. An easier and measurable target such as glycated hemoglobin A1c (HBA1c) and blood pressure in diabetes and hypertension respectively is more possible to adopt as a treatment goal. In lupus, this target is not simple and uncomplicated but regardless we must have a definition of the goal to achieve the best results to which we are striving.
Different definitions of remission in systemic lupus erythematosus
Due to heterogeneity in clinical and laboratory manifestations, SLE requires more complex measures of disease activity. The latest proposed definitions of remission used these composite algorithms based on lupus activity indices, patients’ physical assessment, and glucocorticoid (GC) dosage.
Optimally expressed remission guarantees the patient reduced mortality, prolonged time to next flare or quiescence of the disease, and, most importantly, no organ damage. Complete remission should be our most preferred, but often the most restrictive treatment goal.
Previous longitudinal studies revealed a wide range of prevalence of different definitions of remission [2–4]. Authors reported that those who achieved remission had less lupus activity and a better prognosis over 5–10 years.
Despite a better understanding of lupus pathogenesis, immunology and clinical phenotypes of lupus patients there remain many unmet needs in developing innovative therapies that can potentially limit damage accrual.
However, for safer and more effective treatments to develop, to define treatment goals disease activity assessment tools are needed. In rheumatology in the field of rheumatoid arthritis or psoriatic arthritis, the treat-to-target strategy is used with positive effects, although it has not been proven in lupus.
An ideal therapeutic target is a remission defined and measured by validated tools for assessing disease activity. What does it mean in lupus? What is the most objective tool to consider and how to define remission in SLE? There remain difficult questions.
The discussion about the definition of remission in SLE has been going on for several years. Sustained state without signs and symptoms, quality of life level, fewer flares, lack of immunological activity – what is the definition of the treatment goal?
Different definitions of remission took into account lupus activity assessment indicators such as the SLEDAI score, physical global assessment (PGA), and kind of lupus treatment. These components were used in miscellaneous combinations with various cutoffs and therefore their comparison is impossible in terms of treatment effectiveness, especially innovative targeted therapies in clinical trials.
A cross-section survey of various combinations of definitions of remission was examined by Saccon et al. [5]. These data revealed the most common and the strongest goal in lupus from eight proposed and examined definitions according to the DORIS (definition of remission in SLE) framework [6].
Clinical SLEDAI (clinical Systemic Lupus Erythematosus Disease Activity Index 2000 – cSLEDAI) proved the most achievable goal in lupus in this extremely detailed study. Clinical SLEDAI is a variant of the lupus scale that does not take into account serology results and allows the use of antimalarials, low doses of GCs, and immunosuppressives (ISs) including biologicals.
Despite the lack of clinical activity of lupus, immune activity, i.e. increased antibody concentrations of anti-dsDNA, and decreased C3 and/or C4 complements, may indicate disease activity. Serologically active clinically quiescent disease, well described in the published literature, could be a sustained state without increased risk of flares and damage in summarized evidence [2, 7, 8].
Indeed, being aware of great developments in such fields as immunology and genetics, we are still looking for a biomarker of treatment efficacy, responder to targeted therapy, or predictors of poor outcomes [9]. Abnormal serology in SLE has been discussed by many researchers, and in most studies, immunological activity was not an independent predictor of damage, late morbidity, or mortality [10].
Another aspect of the construction of the final definition of remission was the influence of lupus therapy and its effect on disease activity, the safety of treatment, and the avoidance of dependent complications. Especially GCs and their advantages and disadvantages of chronic use are still discussed. Prednisone doses between 6 and 12 mg per day increase major organ damage by 50% [11].
Our knowledge is growing about increased mortality because of glucocorticoid-dependent damage, and our awareness is growing, which is visible in a significant decrease in mortality in the last decade. The evolution of treatment strategy has become a reality but its goal was not clearly defined.
The final recommendations from the International Task Force appeared in 2021 and constitute a summary of the discussions to date and consensus on a definition of remission in SLE (DORIS) [12]. The recommendations are a guide for clinicians, scientists, and researchers for better defining remission achievement and treat-to-target strategy implementation in patients with SLE.
The treat-to-target strategy improves outcomes in inflammatory diseases and strict targeting in lupus may improve patients’ prognosis in the long term.
Treat-to-target strategy
The experience of the last decade of clinical practice has shown that the treat-to-target strategy led to improved care for patients with inflammatory rheumatic diseases.
The treatment strategy of any disease should be structured and targeted to achieve objectives that deliver long-term benefits. It is obvious that establishing a measurable, efficacious, validated treatment endpoint for assessing remission in a multi-organ disease such as SLE is very complicated.
However, we know that the benefits for lupus patients outweigh any problems or difficulties in long-term perspectives. A goal-directed treatment approach has a profound impact on the management of chronic inflammatory diseases.
Rheumatologists learn from the experience of activity assessment in rheumatoid arthritis (RA). Lessens from RA give the proof that instruments using both clinical judgment and laboratory results indisputably improve outcomes by more effective standard treatment [13].
The fluctuating nature of SLE, relapsing-remitting disease with flares or persistently active disease, leads to irreversible organ damage such as stroke, heart infarction, end-stage renal failure, or glucocorticoid-dependent osteoporosis or cataract [14–16].
Moreover, the quality of life dramatically decreases due to our lack of vigilance and elimination of known risk factors. Both issues, high disease activity level due to poor control with damage progression, and chronic GC treatment with all the consequences, are responsible for the high mortality rate in SLE. Target definition in systemic lupus is expected to help reduce organ damage, improve long-term treatment outcomes and ultimately reduce mortality [17, 18].
The implementation of a treat-to-target strategy in lupus treatment is a very complicated process. It is determined not only by the limits of the remission definition items, but also by less measurable symptoms, pain, arthralgia, or fatigue.
Co-morbidities also affect the patient’s condition or further impair the quality of life of lupus patients, such as depression secondary to disease or during neuropsychiatric manifestations. Management according to the treat-to-target strategy in lupus requires greater clinical knowledge and insight, efficiency, and determination in achieving the goal of disease remission.
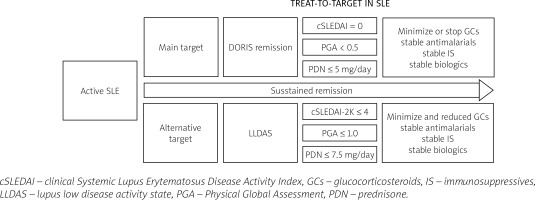
Since 2012, international experts’ work has been underway to define remission as part of the treat-to-target strategy in lupus. Our management and therapy in lupus depend on these essential activities which have become a basis of a treat-to-target strategy (Fig. 1).
Numerous definitions have been explored in terms of duration and reduction in risk of damage, but currently the DORIS (definition of remission in SLE) remission and LLDAS (lupus low disease activity state) appear to be the most reliable and achievable goals [12, 19].
Definition of systemic lupus erythematosus remission, 2021
In 2016 an international task force published a framework for DORIS definition without final recommendations [6]. To meet the expectations in November 2021 the final recommendations were published and a simplified and universal definition was created that can be applied in clinical practice [12].
The International Task Force DORIS described remission as a complex of factors measured by cSLEDAI which does not take into consideration serology results, PGA to reflect the patient’s perspective, and the dose of prednisone (PDN) as a factor affecting the risk of long-term organ damage.
Remission recommended by the DORIS task force is defined by cSLEDAI = 0, PGA < 0.5 points, and PDN ≤ 5 mg per day. The Systemic Lupus Erythematosus Disease Activity Index score is included in the global score system, which provides an overall measure of lupus activity alongside the European Consensus Lupus Activity Measurements SLAM (Systemic Lupus Activity Measure) [20] and, more complicated and difficult to apply, the individual organ assessment British Isles Lupus Assessment Group Index (BILAG) [21].
Evaluation of lupus activity by SLEDAI score requires the application of certain principles, but most of the points of assessment are not objective and depend on clinical judgment. It follows that we do not have independent tools for lupus assessment activity. Similar doubts apply to PGA, which reflects the health-related quality of life from patients’ perspectives.
Nevertheless, remission by any definition is associated with improving quality of life, which is the most important factor for patients with SLE. The principle of lupus treatment for rheumatologists who deal with guiding the patient should be minimizing GCs doses, and this is our direct goal in the definition not only of DORIS remission. Maintaining standard treatment during remission by ISs and/or biologics is necessary and antimalarials are allowed.
The most restrictive definition and most expected clinical condition is DORIS complete remission with no clinical and serological activity (SLEDAI = 0) with PGA below 0.5 points and with maintenance antimalarial therapy without the need for any dosage of GCs and IS or biologics.
The frequency of SLE remission in available data and clinical practice is not high and is still insufficient. In the literature, many propositions of alternative, more achievable goals have been presented.
Remission key messages are shown in Table I.
Table I
Remission key messages
Low systemic lupus erythematosus activity
Multiple studies based on multiethnic and multicenter cohorts evaluated lupus activity according to alternative targets before the DORIS recommendations for remission were published. We have several definitions of low disease activity widely described in the literature.
The term low disease activity (LDA) was defined by Toronto Lupus Cohort investigators and included cSLEDAI ≤ 2 points (without serology results index) and in the most restrictive treatment rules GCs and IS exclusion [22].
The remission objective proposed by Franklyn et al. [23], low lupus disease activity state (LLDAS), is a potential alternative target in our lupus treatment strategy. Among the components of the definition of LLDAS are SLEDAI-2K ≤ 4 points and PGA ≤ 1.0 points, and a minimal dose of PDN ≤ 7.5 mg per day. In remission by LLDAS definition, an additional criterion of any clinical or serological activity must be met.
Due to the level of listed indicators reflecting disease activity, this definition represents a compromise between achievable therapeutic effects and minimizing GC doses. The strongest evidence refers to time-dependent reducing disease flares and organ damage accrual in patients with remission determined by LLDAS definition [23, 24]. Consequently reduced damage progression is associated with decreased mortality by almost 70% as well as improving patient outcomes [25].
Furthermore, based on post-hoc analysis of innovative therapies in SLE, belimumab or anifrolumab, we know that LLDAS has a potential validated outcome measure in clinical trials [26, 27].
Prevalence, predictors and prognostic benefits of remission
Prevalence of remission depends on management and utilized available treatment options and is still an unmet need in the population of lupus patients. In the therapy of systemic lupus, we are aware of the limited effectiveness of possible treatments and problems with prevention flares, persistently active disease, and damage accrual.
Previous studies have revealed that especially the initial years of the disease have implications for the propensity for flare or remission and they are predictive of long-term outcomes. More aggressive treatment for induction, consolidation, and maintenance of the remission state seems to be a good strategy but not so common and not always feasible. In the future perspectives, remission is a state we should strive for because of fewer complications, lower mortality, and better long-term outcomes.
The percentage of remission in different studies varies between 63 and 74.8% of LLDAS according to Tani et al. [28], and 44% in Asian-Pacific countries according to Golder et al. [24]. Different potential definitions and their impact on capturing the remission state and predicting damage by the SLICC/ACR Damage Index were described by Saccon et al. [5].
As mentioned in the introduction, in this Italian multicenter study, a 69.2% remission rate according to the DORIS definition was noted. Various definitions examined in this study were based on single or a combination of remission items such as SLEDAI score, PGA, and PDN doses [6, 12].
The most attainable definition to meet was cSLEDAI, which in BLISS trials, provided with antibodies against B-lymphocyte stimulator (BLyS) in SLE, was shown to be a target and clinical positive endpoint of belimumab treatment. Clinical SLEDAI is only one component of remission assessment by both LLDAS and DORIS definitions. Low lupus disease activity state is easier to achieve than DORIS remission, and it is related to PDN doses but also patients’ self-assessment by PGA.
Discussion about remission with and without treatment, especially GCs, is a very important point of consideration by experts. Complete cure of the disease is an obvious target for management and treatment in any disease, but in SLE, as a chronic disease, it is not reachable.
Although some predictors of damage are non-reversible, such as non-Caucasian ethnicity and older age, high lupus activity level and chronic GCs therapy directly associated with damage are possible to treat and reduce significantly. Moreover, with so much progress in the diagnostics and treatment of SLE, predictive factors help us to adjust the level of the treatment to achieve the best long-term results.
Definitions of remission in SLE and LLDAS remission have been compared in the field of reducing flares and damage progression in various studies [29, 30]. Based on these data, attempts were made to reach a definite conclusion that deeper and longer remission is associated with stronger protective effects against organ damage and its consequences. The remission achieved by maintenance therapy in different published data is in the range of 25–37% [17, 31, 32].
Evidence from observational studies shows that in the populations of South and North America, Asia, and especially Europe, prolonged DORIS remission on treatment was associated with reduced damage accrual [3, 31, 33, 34].
A complete remission requiring no therapy is very rare in both research and clinical practice, and even more unique given the length of remission. In a Canadian study patients with 5 consecutive years of complete remission not requiring medication accounted for 1.7% of the whole cohort [35] and in other analyses did not exceed 7% [3, 4].
Nevertheless, prolonged drug-free remission is very rare and difficult to achieve in studies but also in clinical practice. First of all, the most important goal reduction of damage accrual and increasing time to flare depends on remission duration and its level [5, 4].
Duration of remission was a key point to consider in published data, because of its association with reduced organ damage. In numerous studies published by Zen et al. [3, 36] Caucasian SLE patients were protected against damage after at least two consecutive years in remission but better outcomes in damage accrual were achieved after prolonged maintained remission defined over 5 years.
However, data from the largest US population DORIS and LLDAS remission studies published by Petri et al. [17] have shown that reducing the risk of long-term damage is possible even with a lower percentage of time in LLDAS on treatment.
The difficulties with remission achievement in such a complicated, heterogeneous disease mean that any period of low SLE activity, especially remission, is an achievement with measurable long-term benefits [5, 22].
A review of 41 SLE studies with over 17 thousand patients revealed the percentage of achieving at least one year of maintained remission as 42.4% to 88% and remission predictors associated with lower accrual of organ damage and better quality of life among lupus patients. In this systemic review, the predictive factors older age at diagnosis, lower baseline SLE activity, and absence of major organ involvement were identified; however, positive serology results were negatively associated with remission [37].
Especially prolonged remission is an achievable and desirable target, and we have multiple proofs that are associated with benefits. Consequently, the path to achieving these benefits should be determined by the principles of treatment.
Chronic GC therapy followed by evidence is a major independent predictor of organ damage accrual in addition to typical steroid-dependent complications such as diabetes, osteoporosis, avascular necrosis, or cataract [15, 38].
Nowadays, with so much knowledge on complications of GCs therapy available, restriction of GCs use seems to be the overriding principle. Unfortunately, tapering and withdrawal of GCs before immunosuppressive therapy to protect against flare and organ damage according to recommendations is not a routine good clinical practice everywhere [16, 39, 40].
Achieving long-term remission and the decision to discontinue GCs therapy do not result in an increased risk of flare, interestingly even in lupus nephritis, where decisions to withdraw GCs and ISs could be more predisposing to an exacerbation [41, 42].
A low level of SLE activity according to DORIS remission or LLDAS is the ideal situation to consider tapering or withdrawing treatment after assessment of the time of recent disease flare and risk factors. The physician’s decision depends on clinical judgment and a good patient’s condition; nevertheless, it may not be disturbed by clinically irrelevant symptoms such as arthralgia, pain, or fatigue. Most of the SLE patients after treatment-induced sustained remission discontinued GCs successfully while only a minority of patients had nonsignificant manifestations.
Tani et al. [42] presented the frequency range of 2.4–50% of GCs-free SLE patients in the cohorts reported in the literature. In this study, of patients who achieved remission and had GCs treatment discontinued, flares occurred in 23% of SLE patients at 2 years; by comparison, in the group of patients who required GCs maintenance therapy, most of the patients (69.8%) had at least one flare during 6 years of follow-up. These data revealed the point of going into GCs-free remission even if this goal required effort to achieve it.
Immunosuppressive therapy was a basic treatment in the context of induction and consolidation of remission. According to recommendations, IS discontinuation should be the next step after GCs tapering or withdrawal following disease activity control. Flares after stopping prolonged ISs are not uncommon although antimalarial treatment can reduce the risk and bring benefits.
Hydroxychloroquine (HCQ) is a cornerstone of SLE therapy and a very important factor especially in a discussion about remission. Consistent antimalarial treatment reduced the risk of flares [43] and, more importantly, mortality [44, 45], and during remission is a beneficial and desirable treatment. The definitions of remission allow the use of chloroquine (CQ) or HCQ for disease activity control.
Despite still limited treatment options in SLE, remission regardless of definition is an achievable goal and reducing the risk of damage and progressive destructive disease depends on our awareness of GCs prolonged overtreatment, sustained HCQ, and reasonable immunosuppressive therapy. The best objective decisions for the SLE patient are made in the longterm perspective, taking into account the risks and benefits of the treatment and management including prognostic predictive factors.
Conclusions
The treat-to-target strategy in rheumatology improved outcomes and should be a key to success in the management of SLE patients. The construction of the definition proper of the treatment goal was an achievement in SLE and has the potential for limitation of organ damage accrual.
Reduction of flares and damage accrual is possible with sustained prolonged remission. Remission components, in any definitions of DORIS or LLDAS, revealed reduced overall activity levels, low SLE activity in cSLEDAI assessed by a physician, good condition in PGA score assessed by patients, and a low dose of GCs with basic antimalarial therapy, ISs, and/or biologics.
Successful implementation of DORIS remission and LLDAS definition as a treatment goal allow the adoption of a treat-to-target strategy for greater damage protection in clinical practice. Chronic GCs treatment is the most dangerous predictor of poor prognosis, an independent risk factor of organ damage in the course of SLE.
The aggressive effective treatment of SLE flares by a high dose of GCs and strong ISs in induction therapy, consolidation of clinical improvement, and achieving prolonged, sustained remission is a way for withdrawing GCs and avoiding complications. The deeper and longer the remission is, the stronger is the protective effect, with less morbidity and mortality, and better long-term outcomes.