Introduction
Systemic lupus erythematosus (SLE) is a prototype of autoimmune diseases in which almost all immunological mechanisms may be involved [1–3]. Despite the enormous progress in immunology, the proper pathophysiological mechanisms of the disease are largely unknown and several disease models exist, clearly showing that none of them is universal and explains satisfactorily the pathological mechanisms [4].
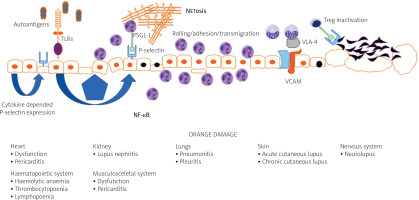
In the course of the disease almost all organs may be affected, including such vitally important as the central (CNS) and peripheral nervous systems (PNS), hematopoietic system, heart, lungs, kidneys, joints and skin [5].
In this review we summarize the role of adhesion molecules as biomarkers in pathogenesis and the clinical picture of SLE with special emphasis on the mechanism that may be at least partially responsible for the development of cardiovascular complications commonly seen in lupus patients.
A systematic search was performed to identify studies on adhesion molecules in lupus using PubMed, Scopus and Google Scholar databases. The following combinations of terms were analyzed: lupus, systemic lupus erythematosus, cellular adhesion molecules (CAM), endothelium, P-selectins, E-selectins, platelet endothelial cell adhesion molecule-1 (PECAM-1), vascular cell adhesion molecule-1 (VCAM-1) and biomarkers.
We considered papers published in English and the systematic search was performed in the period up to December 2021. We included studies on expression of lupus biomarkers both in human and animal models.
Lupus biomarkers and disease activity
The proper assessment of disease activity is of special importance as it may help in treatment-decision making as well as to assess the response to investigational drugs in clinical trials.
For purpose of clinical trials, and to a lesser degree for common clinical practice, the disease activity is measured by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), which is designed to assess the level of the patient’s systemic activity. The scale is as follows:
No activity (SLEDAI = 0).
Mild activity (SLEDAI = 1–5).
Moderate activity (SLEDAI = 6–10).
High activity (SLEDAI = 11–19).
Very high activity (SLEDAI = 20).
However, it is necessary to identify single or multiple biomarkers that may perfectly reflect the disease activity. The characteristics of excellent biomarkers have been recently proposed for urinary biomarkers, but they can be expanded to all biomarkers [6]. In line with this, the ideal biomarker should be:
prognostic, assessing individuals at risk of developing the disease,
diagnostic, helping to make a proper diagnosis of the disease,
predictive of the response to treatment,
pharmacodynamic, determining the optimal therapeutic doses,
a surrogate endpoint [7].
So far, a few candidate biomarkers have been validated in longitudinal cohorts, but none have been used successfully even in the context of clinical trials as they were not superior/equal to currently used biomarkers (complement levels, dsDNA concentration or proteinuria).
Endothelium
The endothelium is a single layer of epithelial cells that line the entire circulatory system from the heart to the smallest vessels. Having a large surface area of approximately 350 m2 and a relatively small total mass of 110 g, it is a key player involved in regulation of vital functions of the cardiovascular system, hemostasis and coagulation, inflammatory and immune responses, vasculogenesis, and angiogenesis [8].
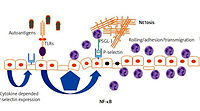
The endothelium plays a major role in the pathogenesis of systemic connective tissue diseases in general and in lupus in particular. Under physiological conditions, the endothelium functions to maintain organ homeostasis through providing the proper vascular tone, precise vascular permeability, and an anticoagulant surface. During autoimmunity, the normal physiological functions of the endothelium are perturbed, contributing to the organ failure characteristic of lupus (Fig. 1).
Vascular endothelial dysfunction is universally observed among patients with SLE, although not all studies confirmed that [9]. It also explains the complicated clinical picture in the disease and may be recognized as a common link between damage of the CNS, kidney, vessels, skin, heart or lungs.
Regardless of the damage in atherosclerosis, the endothelial surface is the place where several immunological mechanisms take place, including antigen presentation, immunocompetent cell rolling and transmigration. Keeping in mind that vessels are almost everywhere in the body, endothelial dysfunction and subsequent damage may be initial steps for lupus-related organ damage (Fig. 1) [10].
In recent years, several non-invasive surrogate markers of endothelial function have been introduced. Among them, flow-mediated dilation (FMD), nitroglycerin-mediated dilation (NMD) and the reactive hyperemia index (RHI) assessed by peripheral arterial tonometry (PAT) are commonly used in clinical research and studies [11].
Unfortunately, they are time-consuming procedures and can only be performed in vascular laboratories, so they are not useful for common clinical practice. Taking into account the importance of endothelium in development and progression of the disease, there is a need for introduction biochemical and immunological markers that may give precise insights into the mechanism of endothelial damage and provide additional clinical data on disease activity.
The endothelium can activate, control, and regulate leukocyte traffic through expression of surface adhesion molecules with concurrent synthesis of chemotactic chemokines after endothelial activation. As a result, immunocompetent cells adhere to the endothelial layer, cross the vessel wall, and accumulate at the site of inflammation [10].
Cellular adhesion molecules
Structure and functions of cellular adhesion molecules
Thousands of membrane proteins serve as cellular adhesion molecules (CAM), which can be categorized as the immunoglobulin super-family of cell adhesion molecules (IgCAMs), selectins, cadherins, integrins, the superfamily of C-type lectin-like domain proteins (CTLDs) and proteoglycans.
Adhesion molecules represent a specific bridge between leukocytes and endothelial cells and can exist in membrane-bound as well as biologically active soluble forms [10].
Based on the place of expression, selectins can be categorized as E-selectin (expressed by endothelial cells), L-selectin expressed on leukocytes, and P-selectin that is expressed both by endothelial cells and platelets. Selectins are important for leukocyte rolling, and P-selectin is also involved in thrombus formation and intravascular coagulation during the immune response [10].
The other role of CAM is to facilitate transmigration of leukocytes across endothelial cell surfaces into extravascular tissues [12, 13].
P-selectin and its regulation in lupus
Pathological endothelial activation caused by inflammatory stimulants for SLE can result in aberrant expression of adhesion molecules resulting in increased leukocyte attachment and infiltration, which directly leads to damage of the vasculature in body tissues [14].
Endothelial damage caused by inflammation or injury can additionally increase expression of P-selectin in platelets, which in turn can activate aggregation and adhesion between platelets, leukocytes, and endothelium [15].
Similar to endothelial cells, platelets are also potent source of P-selectin stored in the α-granules that are quickly translocated to the platelet cell surface upon specific inflammatory stimulation [16].
It has been shown that P-selectin expression by activated endothelial cells and activated platelets is a substantial feature of inflammation-related pathologic states [17].
Taking into account the fact that P-selectin is directly involved in leukocyte-endothelial adhesion, the increased surface expression of this molecule during inflammation makes it an attractive and sensitive biomarker to reveal early pathologic changes and monitor disease treatment [18].
The immunological mechanism leading to over-expression of P-selectin in lupus is not fully elucidated. At least two mechanisms are responsible for increased P-selection expression on the endothelial surface.
Firstly, increased P-selectin gene activity translates directly into P-selectin expression on the endothelium; and secondly, it may be related to cytokine-induced release of P-selectin from Weibel-Palade bodies (WPBs) [19].
At the current level of knowledge it is suspected that in SLE, expression of P-selectin may be regulated via activation of Toll-like receptors (TLRs) with subsequent expression of nuclear factor κB (NF-κB) [20].
Activated endothelium expresses TLR4, TLR2 and TLR3, which have been shown to be important mediators of autoimmunity in lupus [21, 22]. Upon TLR2 stimulation, endothelial cells increase expression of selectins – molecules critical for neutrophil adhesion [22]. Moreover, stimulation of TLR2 upregulates coagulation pathways in human endothelial cells [22].
Similarly to TLR4, TLR2 has also been shown to be important for aortic endothelial cell Weibel-Palade body exocytosis, with subsequent cell-surface expression of P-selectin, resulting in rolling and adhesion of platelets and leukocytes [23].
The same is true as far as the role of TLR3 is concerned. Toll-like receptor 3 is responsible for transmission of inflammatory signals resulting in the expression of inflammatory molecules, such as IFN-γ and soluble E-selectin, in human retinal vascular endothelial cells [24].
The role of P-selectin in lupus may be recognized in the light of endothelial activation and damage. Recently it was found that P- and E-selectin are up-regulated on microparticles and their soluble forms correlated with disease activity.
Moreover, platelets from patients with active SLE preferentially interacted with T regulatory lymphocytes (Treg cells) utilizing the P-selectin/P-selectin glycoprotein ligand-1 (PSGL-1) axis. This leads to inhibition of the regulatory and suppressive properties of Treg cells and particularly follicular Treg cells. As a result, P-selectin engagement on Treg cells limits immunosuppressive and regulatory responses of Treg cells [25].
The role of P-selectins was substantiated in a recent study of Zhang et al. [26], who found P-selectin to be upregulated in lupus nephritis. In line with this finding, blocking P-selectin with specific antibody reduced renal tubulointerstitial fibrosis, renal hypoxia, and peritubular capillary loss. In this study, the authors however did not observe changes of lupus activity indicators, anti-dsDNA antibody, or complement C3.
In agreement with this, a Brazilian study on lupus patients showed higher levels of PECAM-1, VCAM-1, E-selectin, P-selectin, and PAI-1 in SLE patients compared to healthy counterparts.
Moreover, PECAM-1 and PAI-1 can predict SLE with high sensitivity and a specificity. Antinuclear antibody (ANA) titers were significantly and positively associated with PECAM-1, VCAM-1, E-selectin, and PAI-1 levels [27].
The role of P-selectin can also be discussed in a pathophysiological context. P-selectin binds to P-selectin glycoprotein ligand-1 (PSGL-1), a specific ligand expressed on the granulocyte surface. This interaction can promote neutrophil extracellular trap formation, commonly referred to as NETosis [28].
Neutrophil extracellular traps are networks of extracellular chromosomal DNA fibers, histones, and cytoplasmic granule proteins [29]. This brings many pathophysiological consequences as NET formation is directly linked to pathogenesis of many autoimmune disorders including systemic lupus.
It was also established that after activation, platelets and platelet microparticles create complexes with plasmocytoid dendritic cells via P-selectin-PSGL1 interaction and synergize with ICs to induce IFN production, a key pathogenic factor in lupus [30].
More data on the role of P-selectin come from a study on neuropsychiatric lupus (NPSLE) in humans. In this study up-regulation of two adhesion molecules, namely VCAM-1 and P-selectin, in NPSLE patients was observed [31]. This may represent the role of adhesion molecules in initiation and progression of endothelial dysfunction and NPSLE-associated microvascular injury [32].
In spite of the fact that P-selectin is recognized as a valuable marker of vascular diseases (coronary heart disease, stroke, atherosclerosis) [33], the role of this molecule in SLE is still a matter of scientific controversies. High P-selectin levels observed in SLE are rarely corelated with established markers of SLE activity (complement levels and dsDNA concentrations) [26, 27].
At the moment it is unclear whether P-selectin may be used as a marker of disease activity or its role is limited to the clinical situations where vascular involvement plays the crucial role [34].
Immunoglobulin supergene family molecules
The main members of this molecules family are PECAM-1, VCAM-1 and ICAM-1.
Vascular cell adhesion molecule structure and function
Vascular cell adhesion molecule-1 is a cell adhesion molecule member of the immunoglobulin superfamily. Vascular cell adhesion molecule-1 or CD106 is expressed by endothelial cells. It is responsible for the migration and recruitment of inflammatory cells through its interaction with the integrin very late anti- gen 4 (VLA-4) [35].
Vascular cell adhesion molecule-1 consists of several immunoglobulin-like extracellular domains, a trans- membrane region and a cytoplasmic domain, a 19 amino acid carboxy-terminus. In activated endothelial cells, VCAM-1 is expressed in the so-called tetraspanin-enriched microdomains (together with CD9, CD81 and CD151 molecules), which facilitates the expression and function of VCAM-1 on the endothelial surface.
There are two binding variants in human VCAM-1: variant 1 with seven (7d) immunoglobulin-like protein extracellular domains and variant 2 where domain 4 is lacking. Both human variants of VCAM-1 bind to a4b1 integrin (very late antigen-4, VLA-4) and other integrins, such as a4b7 and adb2.
The role of VCAM-1 is to interact with the integrins which are expressed by eosinophils, basophils, lymphocytes, mast cells and monocytes. The force of inter- action between VCAM-1 and VLA is regulated by the level of expression of integrins: when the affinity is low it promotes rolling, whereas high affinity enables firm cellular adhesion to the endothelium.
Several cytokines produced in tissues regulate the expression of VCAM-1. The other factors which can modulate expression of the molecule are shear stress, high glucose level, reactive oxygen species (ROS), oxidized low-density lipoprotein (LDL), and 25-hydroxycholesterol [36].
Vascular cell adhesion molecule-1 is also a part of the innate immune response as its expression is regulated by TLR on endothelial cells. This involves VCAM in the host response against infections but also in autoimmunity [37, 38].
The role of the vascular cell adhesion molecule in lupus
Human studies have found that serum or urine VCAM-1 levels correlated with disease activity in SLE patients [39–42].
Other positive correlations has been found between serum levels of VCAM-1 and anti-dsDNA antibody concentration, SLE disease activity index, and an inverse correlation with serum concentrations of C3 and C4 complement levels [43, 44]. Vascular cell adhesion molecule-1 serum levels were found to be higher in patients with lupus nephritis (LN) than in patients with SLE but without nephritis.
Moreover, VCAM-1 levels also correlate with disease severity in renal biopsies of patients with LN and in some studies with disease activity and SLEDAI components (C3 complement and dsDNA) [45, 46].
Additionally, in a recent study of du Toit et al. [47], VCAM-1 level was found to be higher in patients with active disease (SLEDAI > 12 points) and in patients with LN. This makes a VCAM-1 a valuable marker of diseases activity and response to treatment [48–51].
Of special interest is also the fact that VCAM-1 makes it possible to distinguish active from inactive form of the disease regardless of ethnic origin of the patients, as was recently shown by Stanley et al. [52].
A more detailed insight into the mechanism leading to elevation of VCAM in sera and urine of patients with SLE may be obtained from the animal models of lupus. In murine lupus, VCAM-1 is hyperexpressed in the endothelium, in the glomeruli and in the tubules of MRL/lpr mice, directly showing the endothelial damage as one of the initial steps in lupus-related organ damage [53].
Moreover, as it was established recently in patients with SLE, endothelial activation, measured by high sVCAM-1, was linked to type I IFN activity, representing a potential pathophysiological link between IFN activity and endothelial dysfunction [54].
In 2008 a Scandinavian group reported that, after adjustment for nephritis and disease activity, a high level of sVCAM-1 can help to discriminate a subgroup of SLE patients with previous cardiovascular incidents from SLE patients free from cardio- vascular diseases.
In this study, the levels of sVCAM-1 correlated with TNF-α and its soluble receptors in the patients and controls investigated, clearly showing that lupus activation is driven by the proinflammatory milieu commonly present in lupus patients [9].
Platelet endothelial cell adhesion molecule structure and function
Platelet endothelial cell adhesion molecule-1 is a type I transmembrane adhesion molecule of 130 kDa, which belongs to a subgroup of the Ig superfamily, characterized by the presence of immunoreceptor tyrosine-based inhibitory motifs.
The structure of PECAM-1 is based on extracellular 6-Ig homology domains capable of both homophilic and heterophilic binding [55]. PECAM-1 (CD31) is expressed on platelets, monocytes, and neutrophils, as well as on the surface of endothelial cells, and is responsible for blood and endothelial cell interactions.
The main role of this molecule is to orchestrate cell extravasation, which enables immunocompetent cells to reach the site of inflammation [56]. PECAM-1 plays a double role in inflammation.
Its anti-inflammatory properties include inhibition of leukocyte activation, reduction cytokine synthesis during inflammation, and the maintenance of vascular barrier integrity [57].
Contrary to this, in certain pathophysiological circumstances, PECAM-1 is responsible for trans-endothelial migration of white blood cells (monocytes and neutrophils), and this activity is regulated exclusively by interleukin-1β [58–60].
The role of the platelet endothelial cell adhesion molecule in lupus
A few studies that have investigated the relationship between PECAM-1 levels in patients with SLE found PECAM-1 elevated [27], but this has not been confirmed by the results of all the studies [61].
In the study of da Rosa Franchi Santos et al. [27], PECAM-1 level was found to be elevated and correlated with ANA titer. Moreover, PECAM-1 level was found to predict SLE development with high sensitivity and specificity.
In another study, however, sPECAM level was found to be not different from the control group [61]. Discrepancies in the studies make it difficult to reach a final conclusion, and the role of PECAM-1 as a biomarker of endothelial injury in lupus is not established yet.
In one study it was found that gene polymorphism for PECAM-1 may play a protective role in the development of lupus-related atherosclerosis [61], but the clinical relevance of this finding is largely unknown.
Intercellular adhesion molecule family
The ICAM family consists of five members, named ICAM-1, ICAM-2, ICAM-3, ICAM-4, and ICAM-5 [62].
The ICAM members may be present in membrane-based or soluble forms. Intercellular adhesion molecule-1 (ICAM1/CD54) is a transmembrane glycoprotein of the immunoglobulin superfamily of adhesion molecules expressed on endothelial cells, platelets, and leukocytes [63].
Intercellular adhesion molecule-1 is responsible for adhesion of cells, activation of lymphocytes and trans-endothelial transmigration of leucocytes to sites of inflammation [64]. This function is realized by interaction between ICAM-1 and LFA-1 (lymphocyte associated antigen-1), an integrin expressed on leukocytes. In normal condition endothelial cells express low levels of ICAM-1, but this level rises significantly as the result of endothelium activation [65].
When activated it enables adhesion of immune cells to the endothelium, and stabilizes cell–cell interactions. Intercellular adhesion molecule-1 is critical for transmigration from the circulation into tissues in inflammatory processes [66].
The ICAM-1 gene consists of 7 exons: exon 1 encodes the signal peptide, exons 2–6 the five Ig-like extracellular domains, and exon 7 the trans-membrane and cytoplasmic domains [67].
The role of intercellular adhesion molecule-1 in lupus
Overexpression of ICAM-1 has been observed in several autoimmune diseases [68–72]. Studies on ICAM-1 in lupus patients gave contradictory results. The intercellular adhesion molecule-1 level in blood and urine was found to be elevated [73–77] or unchanged [78].
Recently Guo Liu et al. [79] performed a comprehensive metanalysis on ICAM-1 in patients with SLE. The authors found that both blood and urine ICAM-1 concentrations were higher in SLE patients compared to controls.
Of note is also the fact that that factors such as Asian ethnicity, gender of the patients and type of treatment contributed to the generally higher levels of the molecule. Surprisingly, they did not find any relationship between disease activity and ICAM-1 level. They surmised that although ICAM-1 may be a valuable marker of the disease, its role as a marker of disease activity is limited [79].
Contrary to this, in a recent study Yu et al. [46] found that in LN patients the ICAM-1 level increased during the renal flare and corelated with the level of proteinuria but not anti-dsDNA or C3, nor histopathological features.
Conclusions
We are still some way from accepting any endothelial biomarker as a precise tool to assess the disease activity. At the moment, P- and E-selectin seem to be promising biomarkers in lupus, especially in patients where vascular involvement is present. However. the role of these molecules as universal lupus biomarkers is still a matter of scientific controversies.
The main limitation of this is the fact that although endothelial biomarkers reflect the endothelial activation, dysfunction, and damage, not all forms of SLE go together with clear endothelial damage. This is true for LN, where vessel disease plays a role but due to the diversity of lupus presentation and pathomechanisms involved any simplification may not be free of bias.
More promising data come from studies on VCAM-1, which correlates with laboratory and clinical parameters of SLE activity. However, since most of the data on the role of CAM comes from comes from research on LN and cardiovascular involvement, it is difficult to draw generalized conclusions.
At present, we may accept adhesion molecules as endothelial biomarkers to get some insight into activity of LN or lupus-related atherosclerosis. The results of biomarker levels’ assessment, however, should be interpreted with caution, as they may not necessarily reflect the disease activity.