Introduction
Polymyalgia rheumatica (PMR) is considered to be the most common inflammatory rheumatic disease occurring in older adults [1, 2]. Its onset peaks in the age group 71–80 years and its prevalence increases until the age of 90, with a slight decrease thereafter. Its annual incidence rate is estimated between 0.12 and 2.3 cases/1000 (depending on study design and population) in persons aged 50 years and older [3–7].
At present, no specific laboratory tests are available and PMR diagnosis is essentially clinical. Some criteria have been proposed [8, 9], and they may be useful in everyday clinical practice. The typical presentation of PMR involves a sudden-onset and disabling pain in both the shoulders and pelvic girdle, associated with morning stiffness lasting > 45 minutes.
Pain of the neck and constitutional symptoms can be additional manifestations. Inflammatory markers such as erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) and interleukin 6 (IL-6) serum concentrations are usually raised at the time of diagnosis or when PMR relapses [10–13]. Finally, a fast remission following < 20 mg/day prednisone is commonly used to confirm diagnosis [14, 15].
However, atypical presentations are far from infrequent, and several conditions can mimic PMR [16–21]. For instance, it is not infrequent that some patients diagnosed at first with PMR are reclassified as having a different disease at follow-up. Additionally, normal values both of ESR and CRP at onset do not exclude diagnosis of PMR, if typical features are present and mimicking conditions are carefully excluded [22–25].
Finally, some patients with PMR-mimicking diseases can have a fast response to < 20 mg/day prednisone, but this response is usually transitory [14, 15]. Aetiology and pathogenesis of PMR are debated [26–28]. Polymyalgia rheumatica has been reported as an adverse event following immunization (AEFI) [29, 30].
The primary aim of our paper is to provide an overview of PMR and PMR-like syndromes following the most common types of COVID-19 vaccines, namely mRNA (tozinameran and mRNA-1273) and adenovirus-vectored (ChAdOx1-S) vaccines. Additionally, we aim to discuss whether PMR following COVID-19 vaccines could be considered a true adverse or a coincidental event, as well as exploring the possible pathogenetic mechanisms of this AEFI.
Material and methods
Our review is based on a non-systematic search of PubMed and Medline (COVID interface) performed on January 18, 2022 with the following MeSH terms: [polymyalgia rheumatica AND SARS-CoV-2 vaccines OR COVID-19 vaccines] OR COVID-19 vaccination OR COVID-19 immunization OR tozinameran AND BNT162b2 OR mRNA-1273 vaccine OR adenovirus-vectored vaccine AND ChAdOx1-S.
Concomitant or overlapping giant cell arteritis (GCA) was an exclusion criterion. Each paper’s reference list was scanned for additional publications meeting this study’s aims. When papers reported data partially presented in previous articles, we opted to choose to the most recently published data.
Results
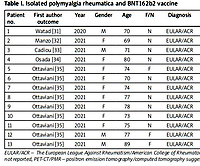
We found 17 case reports where isolated PMR followed immunization with COVID-19 vaccines. Thirteen of these patients received tozinameran (BNT162b2), a nucleoside-modified mRNA vaccine encoding the spike (S) protein for SARS-CoV-2 [31–35]. In Table I, we list the main features of these 13 patients.
Table I
Isolated polymyalgia rheumatica and BNT162b2 vaccine
| Patient no. | First author outcome | Year | Gender | Age | F/N | Diagnosis | US/PMR | PET-CT/PMR | Dose | Time to reaction | Suspected drug | GCs alone | Final |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Watad [31] | 2020 | M | 70 | N | EULAR/ACR | n.r. | n.r. | first | 3 days | none | yes | improvement |
| 2 | Manzo [32] | 2021 | F | 69 | N | EULAR/ACR | yes | yes | first | 1 day | none | yes | improvement |
| 3 | Cadiou [33] | 2021 | M | 71 | N | EULAR/ACR | yes | no | second | n.c. | none | yes | improvement |
| 4 | Osada [34] | 2021 | F | 80 | N | EULAR/ACR | yes | CT | second | 2 days | none | yes | improvement |
| 5 | Ottaviani [35] | 2021 | F | 74 | F | EULAR/ACR | yes | yes | first | 10 days | none | yes | improvement |
| 6 | Ottaviani [35] | 2021 | F | 70 | N | EULAR/ACR | second | 15 days | none | yes | improvement | ||
| 7 | Ottaviani [35] | 2021 | F | 74 | N | EULAR/ACR | second | 14 days | none | yes | improvement | ||
| 8 | Ottaviani [35] | 2021 | F | 77 | N | EULAR/ACR | second | 10 days | none | yes | improvement | ||
| 9 | Ottaviani [35] | 2021 | F | 78 | N | EULAR/ACR | second | 15 days | none | yes | improvement | ||
| 10 | Ottaviani [35] | 2021 | F | 73 | N | EULAR/ACR | first | 10 days | none | yes | improvement | ||
| 11 | Ottaviani [35] | 2021 | F | 75 | N | EULAR/ACR | second | 5 days | none | yes | improvement | ||
| 12 | Ottaviani [35] | 2021 | M | 77 | F | EULAR/ACR | third | 8 days | none | no | improvement | ||
| 13 | Ottaviani [35] | 2021 | M | 89 | F | EULAR/ACR | first | 10 days | none | yes | improvement |
[i] EULAR/ACR – The European League Against Rheumatism/American College of Rheumatology, 2012 classification criteria, F – flares, GCs – glucocorticosteroids, N – new onset, n.c. – not clear, n.r. – not reported, PET-CT/PMR – positron emission tomography/computed tomography suggestive for PMR, US/PMR – ultrasound findings suggestive for polymyalgia rheumatica.
All patients were aged over 65 (between 69 and 89 years old), 9/13 were female, 10/13 had new-onset PMR. There was no autoimmune/rheumatic background. Time to reaction (delay between the day of the vaccine injection and PMR onset) was between 1 and 15 days. In 5 patients, PMR onset followed the first dose. All patients improved with glucocorticosteroids (GCs) therapy alone; only in one patient (patient 12) did PMR follow the third dose and methotrexate was added to 5 mg/day prednisone. Causality evaluation was made for each patient.
Rozin [36] reported a patient 83 years old with PMR following the second COVID-19 vaccination. However, he did not specify the type of COVID-19 vaccine, and the time to reaction (three weeks) seems too long to hypo- thesize a cause-effect relationship. Such a long reaction time (20 and 45 days) was also present in two cases of PMR following the first dose of ChAdOx1-S SARS-CoV-2 vaccine, reported by other investigators [37].
Mettler and co-authors found in Vigibase, the WHO pharmacovigilance database (https://www.who-umc.org/vigibase/), 290 PMR cases among 1,295,482 reports (frequency = 0.022%). One third of them followed adenovirus-vectored vaccines [38].
More recently, the “COVID-19 and autoimmune systemic diseases”, a collaborative network of Italian rheumatologists, reported about a quarter (25.9%) of PMR-like features following immunization with adenovirus-vectored vaccines [39].
On the other hand, to the best of our knowledge, cases of PMR following adenovirus-vectored vaccines are uncommonly published. For instance, a French case series reported only a patient with PMR following immunization with this type of vaccine: he was a 65-year-old patient who developed new-onset PMR 10 days after the second dose of adenovirus-vectored vaccine [35].
Finally, Izuka et al. [40] reported a 70-year-old man who developed PMR-like manifestations 7 days after the second dose of the mRNA-1273 vaccine. According to the authors’ knowledge, this was the first case of PMR-like syndrome following mRNA-1273 vaccination.
Discussion
Since the early 2000s, some researchers have reported PMR as an AEFI following influenza vaccination [29, 30]. To date, PMR following immunization with influenza vaccination is the most common form of PMR as AEFI [41–44].
In line with the World Health Organization (WHO) guidelines, AEFI is “any untoward, unfavorable, or unintended medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine. This adverse event may be symptom, disease, abnormal laboratory finding, unfavourable or unintended sign” [45].
The hypothesis that the link between PMR and AEFI could be an expression of the so-called “ASIA syndrome” (an autoimmune/auto-inflammatory syndrome induced by vaccine components called “adjuvants”) has been discussed [31, 46]. Indeed, some investigators proposed a previous exposure to an external stimulus and the development of some “typical” clinical manifestations (myalgia/myositis, arthralgia/arthritis, pyrexia) as major diagnostic criteria of this syndrome [46]. A genetic background mediated by some human leukocyte antigen B1 (HLA-B1) alleles is crucial [47].
According to our literature search, the onset of PMR/PMR-like syndromes following exposure to adjuvants is very uncommon. For instance, only 4 cases (3 following influenza vaccination) were reported in the 2019 version of the ASIA syndrome international registry, which listed 500 cases. Interestingly, this registry reported no new case of PMR between 2016 and 2019 [48].
Polyethylene glycol (PEG) is present in the lipid film of BNT162b2 vaccine, and is a possible culprit of anaphylaxis reactions to COVID-19 vaccines [49]. However, it is still debated whether PEG can induce an ASIA syndrome. Similarly, polysorbate 80 is an excipient used in the preparation of ChAdOx1-S vaccine whose ability to trigger an ASIA syndrome is still to be proven [50].
Based on the frequency of reported adverse reactions following COVID-19 vaccination, PMR must be considered a very rare (< 0.01%) AEFI according to the WHO guidelines [45]. Yet, the dearth of published case reports is at odds with the hundreds of PMR cases reported in Vigibase.
We recently reported the case of a 69-year-old woman who complained of PMR the day after the first dose of the BNT182b2 vaccine. After > 12 months, no different diagnosis was possible [32]. In the weeks following this report’s publication, we received about 60 emails, mostly from patients or their family members, telling us of post-COVID-19 vaccination symptoms that had been diagnosed as PMR.
However, on careful examination of available data, we could confirm PMR diagnosis only in seven of them. Seventy percent of the PMR notifiers in Vigibase were consumers, non-health professionals, and unknown. Therefore, it is more than likely that the alleged frequency of 0.022% was over-estimated [51].
The case reported by Izuka et al. [40] deserves some consideration. Indeed, the authors diagnosed PMR according to the EULAR/ACR classification criteria and found suggestive PET-CT findings, namely pathological uptake of 18-fluorodeoxyglucose at the bilateral shoulder joints, greater trochanter, interspinous bursa, and ischial tuberosity. Polymyalgia rheumatica clinical manifestations, following the mRNA-1273 vaccine, resolved within a month without GCs treatment. According to our literature search, this possibility does not occur with other types of vaccines.
A final question must be addressed. Is PMR following COVID-19 vaccines a true adverse or a coincidental event? According to the WHO guidelines, a coincidental event is “an AEFI that is caused by something other than the vaccine product, immunization error or immunization anxiety”.
These same guidelines propose a four-step process in order to assess the causality of an AEFI, namely:
Evaluation and assessment of the temporal association between vaccine administration and AEFI.
A plausible time window between vaccine administration and AEFI.
Exclusion of other causes such as drugs taken by the patient or comorbidities.
Evaluation and assessment of the causal association, based on what is currently known from the published literature [45].
In Table II, we discuss the WHO process derived from our search of published cases.
Table II
Causality assessment of polymyalgia rheumatica as AEFI after COVID-19 vaccination, according to our literature search
| Temporal association: always present |
| Plausible time window: time between COVID-19 vaccine administration and onset of PMR was always short or very short |
| Other causes: comorbidities or drugs taken by the patients which could explain the insurgence of AEFI were excluded |
| Strength of the causal association: this is a very relevant point. First we proposed the hypothesis that TLR7 and TLR9 could be the common link between PMR and mRNA vaccines, able to favour over-production of inflammatory cytokines (including IL-6), in genetically predisposed individuals [32]. To date, the pathogenetic mechanisms of PMR/PMR-like syndromes following DNA vaccines is unknown |
Toll-like receptors (TLRs) detect and signal within endolysosomal compartments, triggering synthesis of several cytokines essential for the innate immune response, including IL-6. Intracellular and cell surface receptors are present in the TLR family, where TLR7 and TLR9 are intracellular receptors [52]. The peripheral mononuclear blood cells of PMR patients present increased expression of TLR7 and TLR9, which resolves when PMR is in full remission [53].
In addition, an observational study performed on transcriptional signatures in whole blood of healthy volunteers documented strong activation of TLR signalling after vaccination with the BNT162b2 vaccine [54]. To the best of our knowledge, measurements of TLR7 and TLR9 levels are still lacking in patients affected by PMR following immunization with COVID-19 mRNA vaccines. Therefore, ad hoc studies are required in order to verify this hypothesis, and – in general – the strength of the causal association.
The link between COVID-19 vaccines, TLR7, TLR9, and PMR is possible only within a specific genetic profile. Therefore, the assessment of specific genetic polymorphisms (such as HLA-DRB1*04 alleles) may be the element that “closes the circle”. This hypothesized link, if confirmed, would explain why PMR is a more common AEFI when mRNA vaccines are used. Indeed, DNA vaccines usually stimulate the innate immunity through components able to favour the production of cytokines other than IL-6 [55].
Regarding point 2 (plausible time window), the time between COVID-19 vaccine administration and onset of PMR was usually very short. According to our literature search, it was 3 days in 3 patients, and < 10 days in 9 patients. Vigibase reported a 6-day median time when considering only cases reported by healthcare notifiers. In some reports we found in the published literature, PMR manifestations appeared > 20 days (45 days in one of them) after the COVID-19 vaccine [36, 37]. Could this long time window mean just a coincidence?