Introduction
Rheumatoid arthritis (RA) is a multisystem, chronic, T-cell-mediated disease in which immunological abnormalities result in symmetrical small joint inflammation, articular destruction due to synovitis, and extra-articular organ involvement. In developed countries, the prevalence of RA ranges between 0.5 and 1%. Women suffer 3 times more often than men, and peak incidence occurs between 40 and 50 years of age. An important role in the pathogenesis of RA is attributed to a combination of genetic factors and environmental triggers [1, 2].
The simple disease activity index (SDAI), clinical disease activity index (CDAI), and disease activity score (DAS) are commonly utilized in the monitoring of RA activity. The disease activity score with examination of 28 joints (DAS28) is a measure of RA activity, and the number 28 refers to the 28 joints that are examined in this rating [3]. The original DAS score required the evaluation of 44 joints and the DAS28 is a simplified version of it. Four steps are required to calculate the DAS28:
count all the swollen joints in the set of 28,
count the number of tender joints in the same set,
evaluate the erythrocyte sedimentation rate (ESR) or C-reactive protein (CRP) concentration in the blood,
ask the patient to create a “global health self-report” by marking a spot on a 10-cm-long line indicating patients’ pain from “no pain” to “worst pain”.
It should be noted that there are some difficulties and limitations in the interpretation of DAS28. For example, the score may be misleadingly low if one never has a very high ESR result, or RA can affect the feet, which are not included in the 28-joint count.
On the other hand, when a patient has many tender joints but other inflammation and RA disease activity markers are silent, the score may be misleadingly high. Deciding whether a joint is swollen or tender can be quite problematic and may lead to false variability in the score when assessed by the same person on different occasions or by several people on one occasion. And finally, the last criterion – patient “global health self-report” – is a personal feeling [3]. Due to the subjectivity of some existing criteria and the chance of negative inflammatory test results, searching for other helpful benchmarks in the objective assessment of RA activity seems to be justified.
Rheumatoid arthritis pathogenesis is directed by a network of proinflammatory cytokines, including IL-1β, IL-6, and IFN-γ [4–7]. Milman et al. [4] found a significant correlation between plasma IL-6 concentration and all clinical RA activity measures: the Health Assessment Questionnaire, DAS28–ESR, DAS28–CRP, CDAI, and SDAI. In their study IFN-γ correlated with DAS–CRP and SDAI. Because IL-6 correlated with all clinical disease activity scores, Milman et al. [4] suggest this cytokine as a useful RA activity marker. Another study indicated the association of IFN-γ with the immunopathology of RA, but not with the disease evolution [6]. Brahe et al. [5] also showed correlation of IL-6 levels with DAS28 at baseline. But in their study IL-6 did not reveal consistent predictive value for clinical remission or radiographic progression. This is contrary to the findings of Saeki et al. [8] suggesting that baseline IL-6 levels can predict bone destruction progression in early RA patients.
Interleukin 1β is the master inflammatory cytokine within the IL-1 family. Its role in the aetiopathogenesis and activity of the RA was implicated [7]. It is also a strong stimulator of in vitro and in vivo bone resorption [9]. However, to the best of our knowledge, IL-1β levels in RA patients, depending on the disease activity and severity, have not been studied so far.
Selectin sP mediates leucocytes rolling on the activated blood vessel endothelial cells [10]. The concentration of sP-selectin was significantly higher in synovial fluid samples of RA patients compared to osteoarthritis patients [11]. This indicates its role in inflammatory processes within joints in RA. Also, the blood sP-selectin concentration was increased in RA patients compared to control [12, 13]. Additionally, in RA patients serum sP-selectin significantly correlated with CRP and ESR [10]. Conversely, other studies did not find any correlation between sP-levels and CRP [14, 15] and ESR [14].
Literature data on the utility of circulating IL-1β, IL-6, IFN-γ, and sP-selectin concentration evaluation depending on the activity and advancement of RA seems to be inconclusive. Thus, the initial aim of the study was to test whether IL-1β, IL-6, IFN-γ, and sP-selectin circulating levels could be considered as an objective and quantifiable marker of RA severity and activity. Therefore, we compared serum IL-1β, IL-6, IFN-γ, and sP-selectin concentrations in relation to the DAS28 score and radiographic Steinbrocker classification.
Secondly, IL-1β, IL-6, IFN-γ, and sP-selectin concentrations were correlated with each other and with the following: patient age, the DAS28 score, RA duration, and methotrexate (MTX) dose, as well as with ESR, CRP and fibrinogen concentrations. IL-1β, IL-6, IFN-γ, and sP-selectin concentrations in RA patients were also compared to the values obtained in the healthy individuals.
We do this to check whether chosen cytokines and adhesion molecules play a role in the RA pathogenesis of the current cohort.
Material and methods
The study group consisted of 77 patients (69 females/8 males; median age 62 years, age range 18–80 years) with RA, who matched the American College of Rheumatology and European League Against Rheumatism 2010 criteria [2]. The median RA duration was 3 years (range 1–15 years). The RA study group had 46 patients who were both anti-citrullinated peptide antibody (ACPA) and rheumatoid factor (RF) positive; 52 patients were only RF positive and 19 were seronegative for both ACPA and RF.
Rheumatoid arthritis patients were treated with MTX at a dose of 10–25 mg/week; no biological therapy was used. 51% of patients received nonsteroidal anti-inflammatory drugs (NSAIDs), and the other 49% were treated with steroids by administering methylprednisolone: 27 (35%) patients with a dose of 4 mg/day and 11 (14%) patients with a dose of 6 mg/day. Table I presents clinical and demographic characteristics of the study RA patients.
Table I
Clinical and demographic characteristics of rheumatoid arthritis patients and the control group
The first step of the analysis was to divide the RA patients, based on the DAS28 score, into the following subgroups: patients in remission (DAS28 ≤ 2.4; n = 11), patients with minimal to low disease activity (DAS28 2.5–3.2; n = 12), patients with moderate disease activity (DAS28 > 3.2 < 5.1; n = 30), and patients with high disease activity (DAS28 > 5.1; n = 24). The DAS28 reflects the disease activity and is based on the number of swollen and tender joints, ESR, and visual analogue scale of disease activity (VAS).
In the second step of the analysis RA patients were classified according to the radiographic Steinbrocker functional classification [16] as follows: class I (n = 27; 23 females/ 4 males), class II (n = 21; 19 females/2 males), class III (n = 13; all females), and class IV (n = 16; 14 females/ 2 males).
The control group was made up of 30 healthy volunteers (23 females/7 males; median age 38 years, age range 18–46 years), undergoing their periodical check-ups at the Medical University Hospital Clinic. We selected patients without the following: any rheumatic, systemic, or metabolic disease, malignancies, or acute inflammation.
The study was conducted in accordance with the Helsinki II declaration and was approved by the local Bioethics Human Research Committee (Permission No. R-I-002/132/2013 approved in 2013 and Permission No. R-I-002/200/2015 approved in 2015). We only recruited RA patients and healthy volunteers who gave their informed written consent. Smokers were excluded from the study.
Sample collection and storage
Blood samples were drawn from all patients and healthy subjects in the morning following a fasting period of 8–10 hours. Tubes containing blood collected without anticoagulant were incubated at room temperature for 30 minutes and centrifuged for 15 minutes at 1000 g. The obtained serum was stored at –20°C awaiting further analysis.
Evaluation of IL-1β, IL-6, IFN-γ, and sP-selectin concentrations
Concentrations of IL-1β were analyzed using an ELISA Quantikine® Human IL-1β/IL-1F2 Immunoassay kit (catalogue number: DLB50; R&D Systems Europe Ltd., Abingdon, England) according to the manufacturer’s instruction. The manufacturer of the assay kit referred to the intra-assay coefficient of variation (CV%) as 8.5% at a mean IL-1β concentration of 18.9 pg/ml, SD = 1.6 pg/ml.
Concentrations of IL-6 were evaluated using an ELISA Quantikine® Human IL-6 Immunoassay kit (catalogue number: HS600B; R&D Systems Europe Ltd., Abingdon, England) according to the manufacturer’s instruction. The manufacturer of the assay kit referred to the intra-assay coefficient of variation (CV%) as 4.2% at IL-6 mean concentration of 16.8 pg/ml, SD = 0.7 pg/ml.
Concentrations of sP-selectin were measured using an ELISA Quantikine® Human sP-selectin Immunoassay kit (catalogue number: BBE6; R&D Systems Europe Ltd., Abingdon, England). sP-selectin serum was diluted 20-fold with Sample Diluent according to the ELISA protocol. The manufacturer of the assay kits referred to the intra-assay coefficient of variation (CV%) as 5.1% at a mean sP-selectin concentration of 84 ng/ml, SD = 4.28 ng/ml.
Concentrations of IFN-γ were assayed using an ELISA Quantikine® Human IFN-γ Immunoassay kit (catalogue number: DIF50; R&D Systems Europe Ltd., Abingdon, England). The manufacturer of the assay kit referred to the intra-assay coefficient of variation (CV%) as 4.7% at a mean IFN-γ concentration of 79.2 pg/ml, SD = 3.7 pg/ml.
Statistical analysis
STATISTICA 12.0 PL software (StatSoft Inc., Tulsa, USA) was used to statistically analyze the obtained results. A Shapiro-Wilk test was used to analyze the normal distribution. The Mann-Whitney U test was used to compare 2 independent samples, and the Kruskal- Wallis test was used for the comparison of 3 or more samples. Correlation coefficients were obtained by applying Spearman’s rank method. If not otherwise stated, the values for each given measured variable are presented as median (Me) with 25th and 75th percentiles. Differences were considered statistically significant for p < 0.05.
Results
IL-1β, IL-6, IFN-γ, and sP-selectin concentrations in RA patients compared to the control group
The whole group of RA patients had a higher IL-1β concentration (Me = 0.28 pg/ml; IQs: 0.12–0.45 pg/ml) compared to the control group (Me = 0.13 pg/ml; IQs: 0.03–0.39 pg/ml), but the obtained difference was not significant (p > 0.05). The RA patients showed almost 3-fold higher IL-6 (Me = 3.58 pg/ml; IQs: 1.87–6.74 pg/ml) and sP-selectin (Me = 93.89 ng/ml; IQs: 84.98– 125.84 ng/ml) concentrations compared to the control group (Me = 1.24 pg/ml; IQs: 0.73–1.67 pg/ml, and Me = 31.84 ng/ml; IQs: 24.70–65.20 ng/ml, respectively) (p < 0.001, respectively). Intriguingly, RA patients had an almost 5-fold lower concentration of IFN-γ (Me = 3.57 pg/ml; IQs: 2.70–12.70 pg/ml) compared to healthy control individuals (Me = 17.33 pg/ml; IQs: 16.61–19.32 pg/ml) p < 0.001).
IL-1β, IL-6, IFN-γ, and sP-selectin concentration dependent on the disease activity score with examination of 28 joints
Analysis of IL-1β, IL-6, IFN-γ, and sP-selectin concentrations dependent on the DAS28 score showed that the levels of proteins tested did not significantly differ between the RA subgroups (p > 0.05). Closer inspection of IL-1β concentration dependent on the DAS28 score showed that patients with minimal to low disease activity had the highest protein levels (DAS28 2.5–3.2). We did not find any relevant differences for IL-1β between each particular patient subgroup and control group, except for patients with minimal to low RA activity and healthy subjects (p = 0.018). The highest IL-6 concentration was observed in RA patients with high disease activity (DAS28 > 5.1). The highest IFN-γ concentration showed RA individuals with minimal to low disease activity. The highest sP-selectin concentration was found in patients with moderate RA activity (DAS28 > 3.2 ≤ 5.1) (S1). Moreover, each patient subgroup had statistically different concentrations of IL-6, IFN-γ, and sP-selectin compared to healthy individuals (p < 0.05).
Supplementary materials 1: IL-1β, IL-6, IFN-γ, and sP-selectin concentration dependent on the disease activity score with examination of 28 joints
IL-1β, IL-6, IFN-γ, and sP-selectin concentration dependent on the Steinbrocker classification
Analysis of IL-1β, IL-6, IFN-γ, and sP-selectin concentrations dependent on the Steinbrocker classification showed that the levels of proteins tested did not significantly differ between the RA subgroups (p > 0.05). The highest IL-1β as well as IL-6 concentrations according to the Steinbrocker stages were seen in class II patients. Class I and II patients had almost 4-fold higher IFN-γ concentrations compared to class III and IV functional stages (S2). We did not find any relevant differences for IL-1β between each patient subgroup and the control group. However, each patient subgroup had statistically different concentrations of IL-6, IFN-γ, and sP-selectin compared to the control group (p < 0.05).
Supplementary materials 2: IL-1β, IL-6, IFN-γ, and sP-selectin concentration dependent on the Steinbrocker classification
In the Table II the inflammatory laboratory parameters levels, IL-1β, IL-6, sP-selectin, and IFN-γ concentrations dependent on the DAS28 score and Steinbrocker classification are presented.
Table II
Inflammatory laboratory parameters levels, IL-1β, IL-6, sP-selectin, and IFN-γ concentrations dependent on the disease activity score with examination of 28 joints score and Steinbrocker classification (results are presented as median and interquartiles)
IL-1β, IL-6, IFN-γ, and sP-selectin concentration dependent on the duration of disease
Some authors [10] indicated that RA duration longer than 4 years resulted in a decreased concentration of plasma cytokines. Therefore, in the next step we analysed IL-1β, IL-6, IFN-γ, and sP-selectin concentrations in RA patients with a disease duration ≤ 4 years compared to subjects with a disease duration > 4 years; however, we did not find a statistical difference (p > 0.05). Similar analysis was performed for the following durations: ≤ 1 year vs. > 1 year; ≤ 2 years vs. > 2 years; ≤ 3 years vs. > 3 years, for ≤ 5 years vs. > 5 years, and for ≤ 10 years vs. > 10 years. We did not find any statistical difference for any of the above-mentioned analysis.
Correlation coefficient results
A correlation coefficient was applied to the whole study group of RA patients, whilst in the next step it was dependent on the gender of patients. Firstly IL-1β, IL-6, IFN-γ, and sP-selectin levels were correlated with each other, and secondly with: the DAS28 score, RA duration, as well as with CRP and fibrinogen concentration and ESR in a period of 1 hour.
In the whole RA group, IL-6 positively correlated with the DAS28 score (R = 0.28; p = 0.015), and IFN-γ negatively correlated with sP-selectin (R = –0.28; p = 0.015) and with CRP (R = –0.31; p = 0.004). In RA females, IFN-γ negatively correlated with sP-selectin (R = –0.26; p = 0.033) and with CRP (R = –0.30; p = 0.011). In RA males, IL-6 positively correlated with the duration of disease (R = 0.71; p = 0.048) and with the ESR in a period of 1 hour (R = 0.75; p = 0.031); sP-selectin positively correlated with the duration of disease (R = 0.78; p = 0.020) and with CRP (R = 0.76; p = 0.028); IFN-γ negatively correlated with fibrinogen (R = –0.86; p = 0.006) (S3). We did not find any other correlation coefficient.
Supplementary materials 3: Correlations between studied cytokines and disease activity score with examination of 28 joints, rheumatoid arthritis duration, and inflammatory parameters (C-reactive protein, fibrinogen concentration and erythrocyte sedimentation rate)
In the whole RA group, we also correlated patients age with: IL-1β, IL-6, IFN-γ, and sP-selectin concentration, DAS28 score, RA duration, as well as with ESR, CRP, and fibrinogen values. We found that patients age only positively correlated with fibrinogen concentration (R = 0.44; p < 0.001)
A relationship between MTX administration and circulating levels of cytokines was reported [17, 18]; therefore, we also correlated IL-1β, IL-6, IFN-γ, and sP-selectin concentrations with MTX dose per week. We found that MTX negatively correlated with IL-1β (R = –0.24; p = 0.039) in the whole RA group. In RA males MTX positively correlated with IFN-γ (R = 0.88; p = 0.008) (S4). We did not find any other correlation coefficient between MTX and IL-1γ, IL-6, IFN-γ, and sP-selectin levels.
Supplementary materials 4: The correlation between methotrexate administration and IL-1βand IFN-γ
In the control group we correlated IL-1β, IL-6, IFN-γ, and sP-selectin concentrations with each other and with age. We found that IL-6 positively correlated with sP-selectin concentration (R = 0.54; p = 0.002). We did not find any other correlation coefficient.
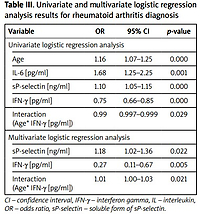
Univariate logistics regression analysis results
In the model of univariate logistics regression analysis, predictor variables influencing RA diagnosis included age, IL-6, IFN-γ, and sP-selectin concentrations. We showed the following if:
the patient’s age increases by 1 year, the chance of having RA increases by 1.16 times (increases by 16%),
the concentration of IL-6 increases by 1 pg/ml, then the chance of having RA increases by 1.68 times (increases by 68%),
the sP-selectin concentration increases by 1 pg/ml then the chance of RA increases by 1.1 times (increases by 1%),
the IFN-γ concentration increases by 1 pg/ml then the chance of RA increases by 0.75 times (decreases by 25%);
the level of the Age* IFN-γ variable increases by 1, the chance of RA increases by 0.99 times (decreases by 0.1%) (Table III).
Table III
Univariate and multivariate logistic regression analysis results for rheumatoid arthritis diagnosis
Multivariate logistic regression analysis results
In the model of multivariate logistic regression analysis, predictor variables influencing RA diagnosis included age, IFN-γ, and sP-selectin concentration. We showed the following if:
the concentration of sP-selectin increases by 1 pg/ml, then the chance of RA increases by 1.18 times (increases by 18%),
IFN-γ concentration increases by 1 pg/ml, then the chance of RA occuring will change depending on the patient’s age, i.e. it will increase by eb2+ b3age times (β2 = –1.28, β3 = 0.02). Table IV presents the interpretation of the interaction (Age* IFN-γ [pg/ml]) in multiple logistic regression analysis.
Table IV
The interpretation of the interaction in multiple logistic regression analysis
Discussion
An objective and well-validated RA activity score, allowing a comparison between serial patient assessments, is still of great interest. Even the most commonly used score, DAS28, has limitations related to ESR and CRP, which would seem to be the only objective components of this rating. Of note, literature data from studies conducted on large cohorts of patients have indicated normal ESR or CRP in RA individuals with active disease [3]. This ongoing problem prompted us to perform our current research.
Our study did not show statistically significant differences between serum levels of the inflammatory markers IL-1β, IL-6, IFN-β, and sP-selectin dependent on the disease activity assessed on the basis of the DAS28 score as well as the severity of the disease assessed based on the Steinbrocker classification. The correlation analysis showed that in the RA group only IL-6 positively correlated with the DAS28 score. However, the concentrations of all molecules tested, except for IL-1β, were statistically significantly different from the control group, suggesting the involvement of IL-6, IFN-γ, and sP-selectin in the course of RA. Univariate logistic regression analysis also indicated that their levels significantly influenced the likelihood of RA diagnosis.
In addition, in patients with RA we analysed markers of inflammation used in routine laboratory diagnostics, i.e. CRP and fibrinogen concentration and ESR value in a period of 1 hour. In contrast to the IL-1β, IL-6, IFN-γ, and sP-selectin analysed by us, ESR and CRP concentrations were significantly different dependent upon the severity of the disease, both between patients divided according to the DAS28 score as well as dependent on the Steinbrocker classification. The fibrinogen concentration was significantly different only between groups of patients divided based on DAS28 score; it was lowest in patients with minimal to low disease activity (DAS28 2.5–3.2) and highest in patients with a high level of disease activity (DAS28 > 5.1). Interestingly, we made similar observations in the case of IL-6 concentration.
In our study we have concluded that the total group of RA patients had higher serum levels of IL-1β compared to control subjects, but the obtained differences were not significant.
Ferraccioli et al. [19] showed that plasma IL-1β, IL-6, and IL-10 concentrations increased mainly in patients with RA duration shorter than 48 months and treated only with non-steroidal anti-inflammatory drugs (NSAIDs), which may explain the lack of statistical differences obtained in our study. Analysis of IL-1β concentration dependent on the DAS28 score revealed that the levels of tested protein did not significantly differ between the RA subgroups compared to controls, except for patients with minimal to low disease activity (DAS28 2.5–3.2).
Moreover, we did not find differences for IL-1β concentrations between the individual Steinbrocker classification stages and control subjects. The IL-1β concentration was also not related to the DAS28 score and radiographic Steinbrocker classification. Altogether, this data may indicate that the above-mentioned cytokine is not a useful circulating biomarker of RA severity and activity. Interestingly, Kokkonen et al. [20] stated that IL-1β and IFN-γ are related to the development of inflammation in pre-arthritis patients. Moreover, it was reported that IL-1β content in the synovial fluid of osteoarthritis patients was higher than that of healthy subjects, and it was positively correlated with patients’ knee joint osteoarthritis score [21].
Based on these data we hypothesize that the material aspect (serum of synovial fluid) is important for IL-1β concentration analysis. Nevertheless, this aspect requires further study.
Pro-inflammatory mediators such as cytokines IL-1β, IL-6, TNF-α, IL-12, and chemokines CCL2 and MIP-1α promote the mobilization of leukocytes to the site of damaged tissue [22]. In the study by Kayakabe et al. [23] the concentration of TNF-α, IL-1α, and IL-6 in the LPS-stimulated leukocytes supernatant was evaluated in both RA patients prior to anti-TNF treatment and in healthy subjects. The authors showed significantly lower levels of TNF-α, IL-1β, and IL-6 in RA patients compared to healthy subjects [23], which is contrary to our results demonstrating a significantly higher concentration of IL-6 in the group of RA patients compared to healthy control.
The current findings are in line with the results of Shrivastava et al. [24], who found that RA patients had significantly higher levels not only of serum IL-6, but also TNF-α and IL-10 as compared to healthy individuals.
In our own studies observing the distribution of IL-6 concentration in individual disease functional Steinbrocker classes, it was found that, paradoxically, the highest median value occurred in class II patients and then only decreased. The lowest IL-6 concentration was revealed Steinbrocker class I patients.
When analysing the concentration of IL-6 dependent on the DAS28 score, the highest value was obtained in patients with high disease activity (DAS28 > 5.1) while the lowest was seen in those with minimal to low RA (DAS28 2.5–3.2), but not as we expected with DAS28 ≤ 2.4, indicating disease remission.
The obtained results clearly show that in our study IL-6 does not maintain linearity in any degree of RA severity and activity. It could be explained by findings of Nishina et al. [17], who observed a significant (p < 0.001) reduction of circulating IL-6 during MTX treatment in early RA patients, which indicates that the applied treatment may have an influence on blood cytokine levels. Interestingly, in their study the post-treatment IL-6 concentration was a strong indicator of radiographic progression [17], which is contrary to our results. However, MTX-dependent changes in cytokine levels differ depending on the study model. For example, Olsen et al. [18] showed that MTX mediated a dose-dependent increase in IL-6 concentrations and IL-6 gene expression levels in human monocytic cell line U937. The current study did not reveal any relationship between MTX treatment and circulating IL-6 levels in the whole group of RA patients, and regardless of patients’ gender.
Rajaei et al. [25] and Shrivastava et al. [24] both showed that serum IL-6 concentration had a significant relationship with RA severity according to DAS28, which was also confirmed in our study. According to the study of Brahe et al. [5], performed on 2 randomized controlled trials, IL-6 serum showed significant correlation with DAS28 in patients with RA duration < 6 months and active disease at baseline (prior any treatment), but IL-6 concentration was not a good predictor of patient’s remission or radiographic progression in 5 year or 2 year follow-up.
From the molecules tested in our study only IL-6 positively correlated with the DAS28 score. It was found that other interleukins also correlated with the DAS28 score, as was revealed for IL-17A by Metawi et al. [26] and for IL-22 by da Rocha et al. [27].
IFN-γ is mainly produced by lymphocytes (B-cells, T-cells, NK-cells) but also by monocyte/macrophage, dendritic cells, and neutrophils [28]. In individuals genetically predisposed to RA development, an increased response of CD45RO+ T-lymphocytes may occur as a result of the presence of unknown exogenous antigens or autoantigens [29]. Activated T-lymphocytes release, e.g., IFN-γ, which inhibits macrophage migration and stimulates them to release pro-inflammatory cytokines: IL-1, IL-6, IL-33, and TNF-α. Increased levels of IFN-γ were found in the plasma and synovial fluid of RA patients [1].
By way of contradiction, our study presented lower IFN-γ levels compared to healthy control individuals. Intriguingly, the current study also showed a negative correlation of IFN-γ with CRP, the increased value of which is an indicator of inflammation [30]. Our study may indicate that serum IFN-γ concentration was negatively influenced by the immune-suppressive mechanisms that prevent excessive inflammation in the course of RA [31].
Kokkonen et al. [20] showed that plasma IFN-γ increases with the progression of RA. Our study is conflicting on this point because we found higher IFN-γ concentrations in patients with minimal to low disease activity compared to patients with moderate and high DAS28 scores. Moreover, class I and class II Steinbrocker functional stages had almost 4-fold higher IFN-γ concentrations compared to patients with more severe RA. However, none of the obtained differences were of statistical significance.
Nasonova et al. [32] and Sigidin et al. [33] showed that anti-IFN-γ and anti-TNF-α showed similar effectiveness in the thickening of synovial membrane in RA. Studies on the animal RA model indicated that administration of IFN-γ antibodies reduced synovial proliferation, cell infiltration, bone damage, and joint cartilage destruction. It also indicated that IFN-γ may hasten RA development in the early stage [28], which is a potential explanation for our findings indicating a higher IFN-γ concentration in less severe cases compared to patients with more severe RA according to the functional Steinbrocker classification.
Increased concentration of serum sP-selectin was found in several connective tissue diseases, suggesting its potential usefulness as a biomarker of connective tissue diseases providing insight into the pathophysiology of rheumatic diseases [10].
In our study, we observed a significantly higher sP-selectin concentration in RA patients, regardless of disease severity and activity, compared to the control group. Similar results were obtained by Littler et al. [12] and Ertenli et al. [13]. Littler et al. [12] in RA individuals also showed a significant relationship between the tested adhesion molecule and CRP and ESR. In our population the sP-selectin concentration positively correlated with CRP only in the case of RA males.
On the other hand, Ateş et al. [10] found a higher serum sP-selectin concentration in RA patients, but it was not significant. However, in their study sP-selectin significantly correlated with CRP and ESR [10], which is in line with the findings of Littler et al. [12].
In the current study we did not find any relationship between severity of disease and sP-selectin concentration. The median concentration of sP-selectin was the highest in the class III functional classification and in patients with moderate disease activity (DAS28 > 3.2 ≤ 5.1). Ateş et al. [10] showed higher sP-selectin concentrations in RA patients with active disease compared to inactive RA, but the obtained difference was not statistically significant. Interestingly, Ertenli et al. [13] found a relationship between plasma sP-selectin concentration and Ritchie articular index and morning stiffness.
Study limitations
The limitations of this study include the lack of IL-1β, IL-6, IFN-γ, and sP-selectin concentrations at baseline, and the number of RA males. Firstly, we did not have patients’ samples before treatment, which made it a retrospective study. Secondly, the correlation coefficient results in males may be affected by the number of cases, and result in an inability to analyse concentrations of proteins tested depending on the RA duration respectively for males and females.
Conclusions
At present, SDAI, CDAI, and DAS are used for the monitoring of RA activity. However, the main disadvantage of applying such scores in clinical practice is the subjectivity of some criteria. Moreover, many patients with active disease may have negative inflammatory tests. Because RA pathogenesis is directed by a network of pro- and anti-inflammatory cytokines, studies targeting a cytokine panel rather than a single molecule as possible effective disease activity biomarkers would be clinically beneficial. Our study is the first that compiles IL-1β, IL-6, IFN-γ, and sP-selectin concentrations in relation to the DAS28 score and radiographic Steinbrocker classification in RA patients.
The conducted study on the current cohort of patients showed that the concentration of IL-6, IFN-γ, and sP-selectin was statistically different from the control group. Univariate logistic regression analysis indicated that their levels significantly influenced the likelihood of RA diagnosis. Among the tested molecules, only IL-6 positively correlated with the DAS28 score, which in general is consistent with other findings. C-reactive protein concentration and ESR value are still playing the dominant role, which in any degree may reflect the RA status. However, we postulate that next to CRP and ESR, also IL-6 could be clinically relevant and may reflect RA activity. Because recently the IL-6 concentration can be determined in applied in vitro diagnostic tests, it presents us with the possibility to test this protein as a marker of RA activity within routine laboratory practice.