Introduction
Juvenile idiopathic arthritis (JIA) encompasses a complex group of childhood rheumatoid diseases caused by some unknown factors and begins before the 16th [1]. Arthritis is a term used to identify inflammation in joints. This disorder is the most common paediatric rheumatic disease [2].
The American College of Rheumatology classified JIA into 3 subtypes, including oligo, poly, and systemic. Also, there are other classification systems of JIA according to the European League Against Rheumatism and the International League of Associations for Rheumatology [3, 4].
Although all factors leading to JIA are still unclear, it is considered a complex disease affected by multiple genes and environmental factors. Some specific alleles of human leukocyte antigen (HLA), such as HLA-A2 and HLA-DRB1, are known to play a role in the aetiology of JIA [5, 6].
Environmental factors affect cells by epigenetic mechanisms, which in the end will influence gene expression. The most well-known epigenetic mechanisms in this procedure are methylation and histone modification [7].
Several studies have shown that the expression of these factors is different in some autoimmune diseases, such as rheumatoid arthritis (RA) [8], systemic lupus erythematosus (SLE) [9], and multiple sclerosis [10].
Deoxyribonucleic acid (DNA) methylation is the primary mechanism of epigenetics and is characterized by the methylation of CpG dinucleotides and the lysine of histone [11]. Several proteins bind to methylated DNA and regulate gene expression by recruiting various inhibitory protein complexes to CpG sites [12].
Methyl-binding proteins (MBPs) are one of those proteins, which have 5 primary members, including methyl- CpG binding domain protein 1 (MBD1), MBD2, MBD3, MBD4, and methyl-CpG-binding protein 2 (MECP2) [13]. Methyl-CpG-binding protein and MBD1 bind to hypermethylated promoters and subsequently recruit repressor complexes to suppress gene expressions.
Interleukin 32 (IL-32), originally named NK cell transcript 4 (NK4), is expressed in the immune cell, including human T cells and natural killer (NK) cells. Interleukin 32 affects several pro-inflammatory molecules like tumour necrosis factor alpha (TNF-α) and interleukin 6 [14]. This gene can be considered a marker of inflammatory diseases.
The maintenance of self-tolerance involves several mechanisms, including primary selection during the evolution of T cells in the thymus. Sometimes immune cells escape from this tolerance, and an adaptive immune response develops against self-antigens.
Tregs play an essential role in preventing autoimmune diseases in humans has been considered as a master regulator or lineage-specific marker of Tregs; therefore, it is essential for the development and maintenance of regulatory T [15].
As has been proposed, disruption of methylation patterns can initiate autoimmune diseases. Methyl-CpGbinding protein 2 and MBD1 are associated with maintenance and regulation of gene expression, and it has been discovered that overexpression of MECP2 enforces forkhead box P3 (FOXP3) to inactivate and suppresses inflammatory response by adjusting the balan-ce of Treg [16, 17].
In this study, we aimed to find the role of the genes in the JIA. Therefore, we chose the FOXP3 gene as a transcription factor that plays a role in the self- tolerance immune mechanism, the IL-32 gene to define the inflammatory state of disease, and the MECP2 and MBD1 genes that play a role in epigenetic mechanisms. Our goal was to find a correlation between MECP2 and MBD1 expression with FOXP3 and determine if FOXP3 expression is controlled by an epigenetic mechanism.
Material and methods
Patients and controls
In this study, we recruited patients with JIA from the division of paediatric rheumatology, who had been already diagnosed and classified into clinical subtypes by a rheumatologist.
Oligoarticular and polyarticular involved the same immune systems mechanisms, so these 2 subtypes participated in this study. Healthy children, whose clinical and preclinical analysis confirmed that they had no disea-se and came to the hospital for a check-up or minor surgical procedures, were considered as a control group.
Fifty-one oligoarticular and 15 polyarticular (66 patients) alongside 60 control individuals were included in the study. Age and gender were matched in patients and the control group. 65% of both patients and controls were female. The patient’s mean age was 10 years, and the standard deviation (SD) was 4.1 years, while the control means age was 9.3 and their SD was 4.9 years (Table I).
Table I
Demographic and clinical characteristics of enrolled individuals
The study protocol was approved by the Shahid Beheshti University of Medical Sciences Ethics Committee (IR.SBMU.RETECH.REC.1394.429; 2016-01-10).
Materials
Lymphoperp was purchased from Inno-Train, Germany (cat no. 002041600). Ribonucleic acid (RNA) extraction kit and cDNA synthesis kit were purchased from Pars Tous Biotech, IRAN (cat no. A101231 and A101161, respectively). SYBR Green Polymerase Chain Reaction (PCR) Master Mix was purchased from Ampliqon, Denmark (cat no. A324406). DNase I enzyme was purchased from Jena Bioscience, Germany (cat no. EN-173S).
Sequences of all genes were taken from the National Centre for Biotechnology Information and Ensemble databases. All the primers were received in the lyophilized form from Cinaclone, Iran.
Sample preparation
After obtaining informed consent, a 3 ml EDTA-anticoagulated blood sample was collected from each study subject. Peripheral blood mononuclear cells (PBMC) were isolated from 3 ml of fresh blood just after the blood was taken using Lymphoperp in each group.
The cells were then washed twice with phosphate-buffered saline. The number of cells was measured using the Neubauer chamber (haemocytometer), and their appearance was examined under a microscope. Then PBMC cells were stored at –80°C.
Ribonucleic acid isolation and complementary deoxyribonucleic acid synthesis
Total RNA was extracted from the collected PBMC in each group by the Total RNA Purification Kit. The resulted RNA was treated with DNase I enzyme to remove the genomic DNA. The quality of extracted RNA levels was measured by 2% gel electrophoresis and OD 260/280 ratio in a NanoDrop™ 2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA).
Also, NanoDrop Devise determined the concentration of extracted RNA. Then up to 500 ng of those RNA molecules per reaction was reverse transcribed to cDNA using the cDNA synthesis kit in a final volume of 20 μl. cDNAs were stored at –20°C.
Primer design
All primers were designed to be able to identify all isoforms of selected genes. For this purpose, OLIGO software version 7.60 and Genscript were used. The quality of the designed primers was evaluated by http://biotools.nubic.northwestern.edu/OligoCalc.html and Gene Runner software. Table II shows the sequence of each primer.
Table II
Primer sequences used for gene expression analysis
Real-time quantitative polymerase chain reaction
Lyophilized primers dissolved in sterile distilled water. The amount of water added to each primer should be included in data provided by the manufacturer. Then the solution must preserve as stock at –20°C.
Finally, the quantitative real-time PCR method was used to quantify the expression level of FOXP3, IL-32, MECP2, and MBD1. Expression was measured on a Rotor gene 6000 Corbett Real-Time PCR System with SYBR Green PCR Master Mix. Glyceraldehyde-3-phosphate dehydrogenase was used as an endogenous control to normalize expression data.
Standard curves were drawn by linear regression using log(C(t)) vs. log(mRNA amount). To examine the presence of contamination, non template controls were included in each run of PCR, which contained all reagents except cDNA. These controls generated a C(t) > 35 (i.e. the message below detection level) in all experiments.
In this assay, thermal cycling conditions were as follows: 95°C for 2 min, followed by 35 cycles at 95°C for 30 s, 55°C for 20 s, and 72°C for 30 s. The reaction mixture of 20 μl final volume contained 10 μl. Real Time Master Mix without Rox, 5 pmol each primer, 20 ng cDNA, and up to 20 μl PCR-grade H2O.
The threshold cycle (CT) method was employed to calculate the relative expression by the formulas as follows:
R = 2– (∆∆CT)
∆∆CT = (CT target – CT reference) healthy – (CT target – CT reference) patient
Statistical analysis
Data were normalized, and Student’s t-test (t-statistic) was used to compare the relative expression of genes between patients and controls. The correlation between expression levels of genes was computed by Pearson’s correlation coefficients. P-values under 0.05 were considered statistically significant. Statistical Package for the Social Sciences (SPSS 26) was used for statistical analysis.
Results
We analysed the PBMC of JIA patients to determine the expression level of FOXP3, MBD1, MECP2, and IL-32 genes with quantitative PCR. The expression levels of the mentioned genes were normalized and then compared with those of healthy children. Our results showed no significant differences between oligoarticular and polyarticular in each gene expression.
Therefore, we take both as one patient group. The relative expression analysis showed that the expression level of FOXP3, IL-32, and MECP2 respectively increased 2.1-fold, 1.2-fold, and 5.3-fold in JIA patients compared to healthy controls, while the expression level of MBD1 decreased 5.2-fold (Fig. 1).
Fig. 1
The expression of forkhead box P3 (FOXP3), methyl-CpG binding domain protein 1 (MBD1), methyl-CpG-binding protein 2 (MECP2), and interleukin 32 (IL-32) genes in juvenile idiopathic arthritis patients. The expressions of FOXP3, MBD1, MECP2, and IL-32 genes were not significantly changed in patients with JIA compared to healthy controls. The relative messenger ribonucleic acid expression of FOXP3, MBD1, MECP2, and IL-32 genes were examined in peripheral blood mononuclear cells of young patients with juvenile idiopathic arthritis and healthy controls using quantitative real-time polymerase chain reaction.
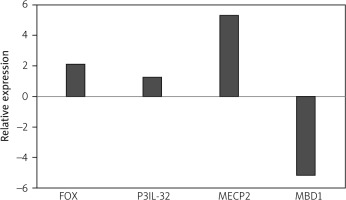
Statistical analysis showed that the change in expression of all the mentioned genes was not significant (p-values = 0.399, 0.0781, 0.225, and 0.082 for FOXP3, IL-32, MECP2, and MBD1, respectively).
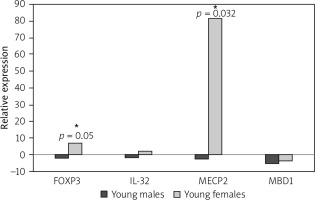
The number of young females suffering from JIA is greater than that of young males. Based on this fact, we separated JIA patients and healthy controls by their sex to analyse the gene expression in each gender separately. Our results showed that the MECP2 and FOXP3 expressions were increased significantly in affected young females compared to healthy young females (p-value = 0.002 and 0.05, respectively).
Our results showed that the expression level of MECP2 and FOXP3 was enhanced 82.1 and 7-fold in affected females, respectively. While affected males showed no significant decrease in expression 2.9-fold for MECP2 and 2-fold for FOXP3 (p-value = 0.2 and 0.17, respectively, for a level of relative gene expression).
In affected males, the expression level of MBD1 and IL-32 was reduced 5.3- and 2-fold, respectively, which were not significant (p-value = 0.07 and 0.21 respectively for relative gene expression).
The expression level of IL-32 was increased in affected females 2.36 fold, while MBD1 expression decreased in affected young females 3.8-fold (p-value = 0.14 and 0.06, respectively, for relative gene expression) (Fig. 2).
Fig. 2
Forkhead box P3 (FOXP3) and methyl- CpG-binding protein 2 (MECP2) are significantly upregulated in affected young females. Juvenile idiopathic arthritis patients and healthy controls are separated based on their gender, and the expressions of FOXP3, methyl-CpG binding domain protein 1, MECP2, and interleukin 32 in affected young males and females were compared with healthy individuals.
*Signs for significant changes.
Our results also revealed that healthy young females express lower FOXP3 compared to healthy young males, but this difference was not significant (p-value = 0.15).
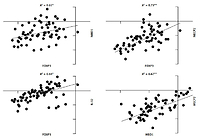
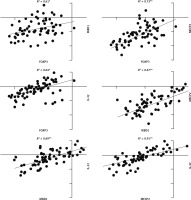
Pearson correlation analysis was used to analyse the correlation between FOXP3, MBD1, MECP2, and IL-32 genes. The correlation coefficient between all genes in JIA patients was significant (p-value < 0.05), and scores ranged between 0.55 and 0.97 (Fig. 3).
Fig. 3
Correlation analysis between gene expression in juvenile idiopathic arthritis patients. There is significant correlation between all analysed gene expression levels.
*Correlation is significant at the 0.05 level (2-tailed), **correlation is significant at the 0.01 level (2-tailed).
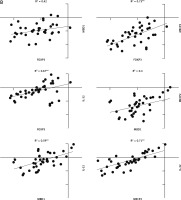
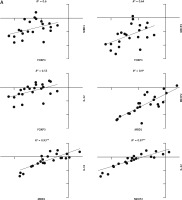
The correlation coefficient in young males with JIA was significant between MECP2, MBD1, and IL-32 (Fig. 4A). However, the correlation coefficient in young females with JIA was significant between all genes with others except MECP2, FOXP3, and MBD1 (Fig. 4B).
Fig. 4A
The correlation coefficient in males and females with juvenile idiopathic arthritis between studied genes. Forkhead box P3 (FOXP3) expression level is not significantly correlated with all other genes in young, affected males.
*Correlation is significant at the 0.05 level (2-tailed), **correlation is significant at the 0.01 level (2-tailed).
Discussion
Children with JIA are at the greatest risk for several complications such as uveitis, nutrition, growth, depression, disturbance, and osteoporosis [18]. Therefore, finding a unique mechanism or a specific cure to reduce side effects is significant. Our patients were treated with methotrexate (MTX) and vitamin D. Also, most of the young females included in this study were in or around the age of puberty.
Our data indicated that MBD1 expression was decreased in both affected young males and females. However, MECP2, FOXP3, and IL-32 were increased in affected young females and decreased in affected young males. The limited number of JIA patients was an important issue, which means there were not enough patients in each JIA subgroup, each gender, and each age. Therefore, we were unable to analyse each of them properly.
Some studies have shown the overexpression of IL-32 in RA and its correlation with the severity of the disease [19]. We observed that the IL-32 expression was upregulated in affected young females but downregulated in affected young males.
Previous studies showed that MTX could reduce the expression level of ILs [20, 21], as we observed in affected young males. Gender hormones such as progesterone suppress the MTX effect and increase IL expression levels [22–24].
In agreement with this, our results showed upregulation of IL-32 in affected females. We believe that these sex hormones are essential contributors to the enhancement of inflammatory factors, and they can be the cause of higher JIA incidence in young females.
Methyl-CpG binding domain protein 1 isoforms contain 2 or 3 cysteine-rich CXXC domains binding to methylated CpG sites [25] and recruit several repressor proteins including MCAF1, HDAC3, and MPG, which can induce gene silencing either by binding to methylated DNA and blocking the transcription directly or by bringing transcriptional histone deacetylase-containing corepressor complexes to methylated DNA templates [26].
Methyl-CpG binding protein 2, the first identified member of MBPs, binds to 5-hydroxymethylcytosine (5 hmC) and 5-methylcytosine (5 mC) residues [27], and acts as a global silencer [28]. Previously we showed that DNA methyltransferases (DNMTs) are downregulated in JIA patients compared to healthy children [29], which can affect the expression of MBPs.
In this study, we observed that MECP2 expression was upregulated significantly in affected young females. This upregulation was reported in several other autoimmune diseases such as SLE and RA [30, 31].
Also, we observed different expression levels of MECP2 between genders, as in previous reports in mice [32]. Additionally, MBD1 expression was reduced in both young male and female JIA patients.
Forkhead box P3 binds to more than 700 genes and changes their expression [33]. Several studies have shown that in FOXP3 -knockout mice, Treg reprogramed to pathogenic helper T (Th) cells, which causes severe autoimmune and/or inflammatory diseases [34].
In our study, FOXP3 was increased totally but not significantly. We separated affected young female patients and male patients, and then analysis showed that FOXP3 expression was increased significantly in affected females, while the expression of this gene was reduced in affected young males.
Cribbs et al. [35] in 2015 and Avdeeva et al. [36] in 2020 confirmed that upregulation of FOXP3 can be caused by MTX, as previous studies showed that MTX could restore Treg activity and upregulate FOXP3 expression in RA. Also, sex hormones can upregulate the FOXP3 expression level.
Previous studies showed that hormone levels in affected young females are higher than in healthy females, which can cause FOXP3 upregulation [37]. These hormones can increase the expression level of FOXP3 in affected females. Interestingly, it could be associated with the cause of mild JIA in females.
Moreover, in young healthy females, the expression of FOXP3 is less than in young healthy males, but not significantly. Due to the FOXP3 role in Treg, the lower level of FOXP3 expression in healthy females can determine why females are suffering from JIA or other autoimmune diseases more than males. According to the description above, the FOXP3 factor can be used as a new prognostic marker in JIA.
Pearson correlation analysis determined that there was a very strong correlation between MECP2, MBD1, and IL-32 in young, affected males, and based on down-regulation of them, probably MBPs do not control the expression of IL-32 through CpG islands in young affected males.
There was a very strong correlation between MECP2 and IL-32, and FOXP3, and based on overexpression of MECP2, FOXP3, and IL-32 in young, affected females, probably MECP2 does not control the expression of FOXP3 and IL-32 by CpG islands in young affected females.
Meanwhile, down-regulation of MBD1 and its strong correlation with IL-32 can be the reason for overexpression of IL-32 in young, affected females.
Conclusions
Our data reveal different expression patterns in JIA in male and female patients, which means that each of them requires a different treatments strategy.
In agreement with our previous work about DNMTs, MECP2 and MBD1 are downregulated in young, affected males, which could lead to epigenetic changes in those patients and is a possible reason for severe JIA in males.
This study showed that the level of the FOXP3 expression in young healthy females can be the cause of their having JIA more than healthy males.
Also, our results demonstrated that the expression level of FOXP3 in young males could not be normalized by MTX. We believe that other FOXP3 regulatory strategies are needed as a promising therapeutic strategy for the treatment of JIA in young males.