Introduction
Arthritis is a broad term that includes a group of more than 100 diseases that affect the joints, including the most common degenerative arthritis, i.e. osteoarthritis (OA), and the most common inflammatory arthritis: rheumatoid arthritis (RA) [1].
Osteoarthritis, a chronic degenerative joint disease, is one of the leading causes of global disability. Although it can affect any joint, most of the OA burden is attributed to knee, hip, hand, and spine OA [2]. It can cause chronic pain, stiffness, and swelling, and, in its latest stage often require joint replacement surgery [1]. Osteoarthritis represents a major public health challenge, with significant implications for affected individuals [3].
Rheumatoid arthritis is a progressive chronic autoimmune disorder characterized by synovial inflammation. It affects most commonly the hand, wrist, and foot, but it can also affect large joints. Rheumatoid arthritis results in musculoskeletal pain, swelling, stiffness, joint and bone destruction, increased risk of developing osteoporosis, and deformity that results in progressive disability but also systemic complications for example, cardiovascular diseases [4].
Although the pathological mechanisms involved in OA and RA are different, the onset and progression of both diseases are associated with several analogous clinical manifestations, inflammation, and immune mechanisms, that is, the migration of immune cells into the synovium of affected joints [5, 6].
The articular cartilage consists of chondrocytes and extracellular matrix (ECM), made out of two main components: type II collagen fibrils and aggrecan proteoglycans [7]. The ECM of a healthy cartilage is in a state of dynamic equilibrium with a balance between the synthesis and degradation of collagen and aggrecan.
This balance is disrupted in both OA and RA in favor of proteolysis, leading to pathological cartilage destruction. Matrix metalloproteinases (MMPs) synthesized by chondrocytes and synovial fibroblasts are considered the main enzymes responsible for this degradation [8]. Several studies have shown that MMP expression is elevated in cartilage and synovial tissues in OA [9–11] and RA [12–14].
The number of total hip arthroplasties in Poland from 2005 to 2019 doubled, from 26,082 to 59,306, and the number will continue to increase according to demographic projections [15]. Most of these cases are due to OA and RA, despite the advances in RA treatment including the use of biologic disease-modifying antirheumatic drugs (bDMARDs).
Current treatments of OA and RA do not inhibit disease progression, while the number of affected patients is increasing. Therefore, treatments targeting MMPs offer a promising approach to this problem and, in recent years, there have been major developments in MMP-targeted treatment related to the genetic background, including the discovery of new synthetic and natural molecules, and potential biomarkers.
This is a state-of-the-art review regarding the role of MMPs in OA and RA, as well as the possible methods of targeting MMPs to alleviate the degradation processes taking part in OA and RA.
Material and methods
The key terms: rheumatoid arthritis, osteoarthritis, matrix metalloproteinases, and tissue inhibitors of metalloproteinases were searched in MEDLINE, ClinicalTrials.gov, and ScienceDirect. Preference was given to the sources published within the past 6 years. After searching the databases electronically, two independent reviewers performed sorting of the articles by relevance to the aim of the review.
The role of matrix metalloproteinases in osteoarthritis and rheumatoid arthritis
Matrix metalloproteinases
Matrix metalloproteinases are a family of calcium-dependent endopeptidases with a zinc-binding active side. More than 20 different MMPs are classified according to the particular substrates they degrade: collagenases, stromelysins, gelatinases, matrilysins, membrane-type MMPs, and others.
Moreover, MMPs release growth factors from carrier proteins, inactivate proteinase inhibitors, and influence inflammatory cytokines and chemokines that can also be responsible for joint destruction [8]. In the human body, MMPs are synthesized in leukocytes, macrophages, endothelial cells, and connective tissue cells such as chondrocytes and synoviocytes, both found in the knee joint.
Matrix metalloproteinases are secreted as pre-proenzymes into the extracellular fluid, where they can be activated by a variety of substances: epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), tumor necrosis factor-α (TNF-α), interleukin-1 (IL-1), and other MMPs.
Conversely, steroid hormones, transforming growth factor-β (TGF-β), plasma proteins such as a2-macroglobulin and a1-antitrypsin, and tissue inhibitors of metalloproteinases (TIMPs) can inhibit MMP activity [16].
The role of matrix metalloproteinases in osteoarthritis and possible matrix metalloproteinases targeted treatments
Pathogenesis of osteoarthritis
Pathologic changes that occur in OA affect the whole joint and include degradation of the articular cartilage, variable degrees of synovial inflammation, thickening of the subchondral bone, or, in other words, remodeling, hypertrophy of the joint capsule, and osteophyte formation. In addition, periarticular nerves, the bursa, and local fat pads may be affected [17].
The onset of cartilage destruction is due to mechanical stress, which either directly damages chondrocytes or activates them to produce abnormal levels of MMPs and reactive oxygen species (ROS) responsible for cartilage breakdown and the release of microcrystals, osteochondral fragments, and products of ECM degradation into the joint cavity.
These fragments stimulate inflamed synovium cells (synoviocytes, macrophages, lymphocytes) to secrete cytokines, chemokines, and lipid mediators, as well as more ROS and MMPs, which can directly degrade the components of the cartilage matrix or dysregulate the metabolism of chondrocytes, leading to an imbalance between cartilage matrix degradation and synthesis. Cartilage breakdown products and pro-inflammatory mediators released by chondrocytes amplify SM inflammation, creating a vicious cycle [18].
Loss of ECM elements such as proteoglycans and type II collagen on the cartilage surface leads to increased water content and loss of tensile strength as the lesion progresses [19]. Inflamed synovium arises from the increased migration of immune cells (macrophages, B lymphocytes, T lymphocytes, and neutrophils) in the subintimal layer [20].
The key MMPs that participate in the degradation of ECM are MMP-1, -2, -3, -9, and -13, demonstrated by animal models, and chondrocytes taken from OA-affected patients [9–11].
Tissue inhibitors of metalloproteinases in osteoarthritis
Tissue inhibitors of metalloproteinases (TIMPs) can directly inhibit ECM proteolysis, as well as indirectly controlling ECM turnover through the regulation of cytokines, chemokines, and cell surface proteins [21].
The imbalance between MMP and TIMP activity has been proposed to be related to the destruction of ECM in OA [22]. Recent findings demonstrate that TIMP levels change as OA progresses, thus some TIMPs are more crucial in one stage of OA than another [23].
Proinflammatory cytokines in osteoarthritis
Proinflammatory cytokines secreted by chondrocytes, fibroblasts, and immune cells are mediators of the metabolic imbalance and they enhance catabolism by stimulating MMP synthesis in OA-affected joints [24]. Among the many representatives of this group, the greatest importance is attributed to interleukin-1β (IL-1β) and TNF-α [25].
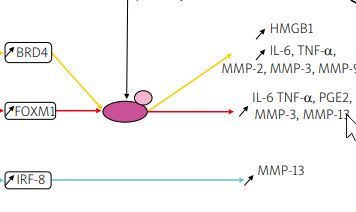
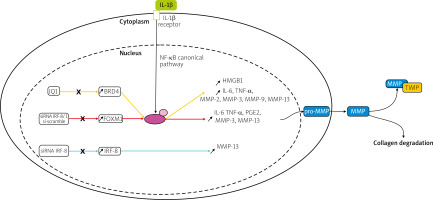
Transcription factors such as forkhead box M1 (FOXM1), interferon regulatory factor-8 (IRF-8), and bromodomain-containing protein 4 (BRD4) were upregulated in IL-1β induced chondrocytes in an in vitro model or human chondrocytes taken from OA patients undergoing total knee arthroplasty [26–28].
Knockdown of FOXM1, and IRF-8, and inhibition of BRD4 using JQ1, an antagonist of BRD4, suppressed the production of inflammatory cytokines, inflammatory mediators, and MMPs, including MMP-3, -9, and -13, shown in Figure 1. This research indicates that silencing transcription factors in chondrocytes can have a protective effect on the joint and therefore could be a potential target for future OA therapy.
Fig. 1
Pathways of potential therapeutic targets. Overexpression of the transcription factors: BRD4, FOXM1, and IRF-8 was observed in IL-1β induced chondrocytes. In studies of BRD4 and FOXM1, it has been suggested that the effect of IL-1β is regulated via the NF-kB signaling pathway, contrary to IRF-8. Matrix metalloproteinases genes are transcribed, translated and in the cytoplasm, pro-MMPs are synthesized. Then they are released into the ECM, converted into MMPs, and either participate in collagen degradation or are inhibited by TIMPs.
NF-κB – nuclear factor κ-light-chain-enhancer of activated B cells, P – phosphate, p65 – transcription factor p65.
To date, no known possible FOXM1 suppressors in OA have been investigated. Currently, potential FOXM1 suppressors are being investigated mainly in cancer treatment research, e.g. in a U2OS cell line (cancer-derived cell line) [29] and in breast cancer cells [30, 31]. Meanwhile, BRD4 inhibition treatment has been shown to have anti-RA effects both in vitro and in vivo [32, 33].
The molecule JQ1 is a selective BET inhibitor, which can displace BRD4 from chromatin. Models of many cancers, e.g. medulloblastoma, breast, and lung cancer showed an anti-tumorigenic response to JQ1 in vivo and in vitro [34–36].
Unfortunately, JQ1 has a short half-life of one hour, which makes its effect short-lasting [37]. The molecule JQ1 analog TEN-010 (JQ2) with better pharmacological properties has been developed and only this analog has been undergoing clinical trials in patients with AML, myelodysplastic syndrome, and solid tumors, but none in patients with OA [38, 39].
Lastly, a study from 2018 treating rats with OA using the TNF-α inhibitor (CAS 1049741-03-8) downregulated the expression of IL-1β, interleukin-17a (IL-17a), IL-8, and MMPs, e.g. MMP-3 and MMP-9 in synovial fibroblasts isolated from rats with OA [40].
The study indicated that treatment with the TNF-α inhibitor could have a protective effect on an OA-affected joint and decrease inflammation, and hence pain for patients suffering from OA.
Ghrelin – a possible treatment in osteoarthritis
Ghrelin is an anti-inflammatory neuropeptide [41], which takes part in stimulating gastric acid secretion and bowel motility, glucose metabolism regulation, sleep, stress and anxiety modulation, taste sensation, and the reward system, and prevents muscle atrophy [42].
Ghrelin can inhibit the apoptosis of chondrocytes, downregulate the expression of MMP-13, and maintain the expression of ECM [43]. Ghrelin attenuates cartilage degeneration, which may have implications for future treatment strategies of OA.
On the other hand, one must note the possible adverse effects of ghrelin, which is an already existing neuropeptide in the body that mainly stimulates appetite and obesity is one of the risk factors of OA onset [44].
However, a possible obstacle to this therapy is ghrelin’s half-life. Ghrelin has a short half-life, which can limit its oral bioavailability leaving only invasive administration as a viable option [42].
Novel synthetic molecule in osteoarthritis treatment
It was found that 2-(8-methoxy-2-methyl-4-oxoquinolin-1(4H)-yl)-N-(3-methoxyphenyl) acetamide (3-B2) down-regulated mRNA expression of MMP-13 and MMP-3 in IL-1β-induced OUMS27 cells without causing serious cytotoxicity [45].
Furthermore, combined treatment with 3-B2 and betamethasone had a synergistic effect and significantly attenuated MMP13 expression. Nonetheless, the study was conducted in vitro, and further in vivo research in the form of clinical trials needs to be undertaken.
It might take quite a long time for clinical trials to start because using broad-spectrum MMP inhibitors has been restricted. Patients tend to develop musculoskeletal syndromes such as arthralgia and myalgia. In conclusion, a potential compound of interest that may help attenuate MMPs in OA-affected joints was identified, but it is a long way from clinical trials.
Intra-articular injection of hyaluronic acid with iron-glutathione complex
Since there is no established disease-modifying agent for OA, there are many treatment options that focus on alleviating OA symptoms. Among these options is the intra-articular injection of hyaluronic acid (IA-HA), specifically into the knee and shoulder [46].
An injection of HA with iron-glutathione complex, when incubated with a synovial fluid (SF) sample collected from a late-stage OA patient, had lower MMP activity, including MMP-1, -2, -3, -7, -8, -9, and -13, when compared to the subcutaneus injection of 2% sodium salt of hyaluronic acid-treated SF group.
It was well tolerated at lower doses by chondrocytes, meaning there was no significant difference in cellular activity and cell proliferation was not affected [47]. This hydrogel is a possible mediator of MMP regulation for OA therapy.
MicroRNAs in osteoarthritis
MicroRNAs (miRNAs) are endogenous small noncoding 18 to 24-nucleotide-long fragments of RNA, which regulate gene expression at the post-transcriptional level by inhibiting translation and/or by promoting RNA degradation [48].
They take part in many biological functions: cell differentiation, inflammation, embryogenesis, metabolism, organogenesis, and apoptosis [49]. Both inflammation and apoptosis are involved in cartilage homeostasis, and therefore OA, which seem to be regulated by miRNAs [50, 51]. MicroRNA-203a, miRNA-103, and miRNA-122 levels were significantly upregulated in cartilage from OA patients, who had undergone total knee arthroplasty [51–53].
Overexpression of these mi-RNAs increased ECM degradation, the inflammatory response, and MMP-13 expression in chondrocytes. Molecular mechanism analysis revealed that miRNA-203a targets Smad3, miRNA-103 sphingosine kinase 1 (SPHK1), and miRNA-122 sirtuin 1 (SIRT1).
In conclusion, these studies provide insight into the pathogenesis of OA and potential OA treatments. Possible therapy for OA could target either the miRNAs or the molecules they affect.
Long non-coding RNAs in osteoarthritis
Long non-coding RNAs (lncRNAs) are a class of non-coding RNA with a length greater than 200 nucleotides, that can regulate gene expression at various levels, including transcriptional gene silencing, mRNA maturation, transport, and alternative splicing [54].
When human knee-chondrocytes were transfected with lncRNA small nucleolar RNA host gene 1 (SNHG1) expression of pro-inflammatory cytokines and MMPs, including MMP-1, -3, and -9 decreased, and ECM degradation increased. MicroRNA-16-5p was the direct target of SNHG1 [55].
In contrast, lncRNA plasmacytoma variant translocation 1 (PVT1) was elevated in chondrocytes taken from OA patients, who had undergone total endoprosthesis surgery. When PVT1 was muted expression of inflammatory cytokines, and MMPs, including MMP-3, -9, and -13 was suppressed and ECM degradation increased. Plasmacytoma variant translocation 1 could directly bind to miRNA-149 and repressed its expression and activity [56].
Altogether, this research confirms that lowering the expression of PVT1 and miRNA-16-5p using SNHG1 may ameliorate the progression of OA by alleviating the ECM aberrant catabolism and inflammation, hence it is a promising therapeutic strategy against OA. So far no clinical trials have started where lncRNA has been implemented as therapy, even for other indications.
Other matrix metalloproteinases-related developments in osteoarthritis
Matrix metalloproteinases-generated neoepitope of C-reactive protein – a potential osteoarthritis biomarker
A study from 2021 demonstrated the capability of the MMP-generated neoepitope of C-reactive protein (CRPM) to be a biomarker of local and systemic inflammation in knee OA [57].
Matrix metalloproteinases-generated neoepitope of CRP is generated by MMP-cleavage in inflamed tissues [58]. The advantage of CRPM compared to CRP is that it is independent of BMI because OA is a disease associated with high BMI [59].
Gene polymorphism and osteoarthritis susceptibility
Matrix metalloproteinases-1 expression is affected by a single nucleotide polymorphism (SNP) in the promoter of the MMP-1 gene in chromosome 11. The association of 1G/2G at -16071 (rs1799750) with OA susceptibility has been studied before with conflicting results in several populations (Turkish, Egyptian, Greek, and Chinese) [60–63]. According to the most recent findings, the 2G/2G genotype and 2G allele carriers of MMP-1 gene rs1799750 polymorphism increased the risk of OA in the Chinese Han population [64].
At the moment more studies point to an association between the rs1799750 SNP and OA susceptibility. Despite that, studies using more diverse ethnic populations are necessary to confirm this association.
Another SNP that is associated with an increased risk of OA is the MMP-13 gene (rs2252070). The expression of MMP-13 is affected by 77A/G polymorphism in the promoter of the MMP-13 gene. Matrix metalloproteinase-13 rs2252070 (-77G > A) mutation is associated with knee OA susceptibility, increased disease severity, and up-regulated MMP-13 levels in the Chinese Han population [65].
In addition, clinical trials for 77A/G polymorphism of MMP-13 and risk for developing knee OA are currently being conducted in the Greek population [66].
The role of matrix metalloproteinase in rheumatoid arthritis
Pathogenesis of rheumatoid arthritis
The pathogenesis of RA is based on the fact that the patient’s body reacts to autoantigens, e.g. citrullinated peptides, or a foreign peptide, e.g. a viral or bacterial peptide that is cross-reactive with an autoantigen [67]. Activated T cells, B cells, and monocytes infiltrate the synovial membrane in joints, as that is where the autoantigens accumulate.
Cytokines and chemokines secreted by leukocytes, such as tumor necrosis factor, IL-6, and granulocyte colony-stimulating factors to name a few activate endothelial cells, stimulate neovascularization and leukocyte migration, and cause expansion of synovial fibroblast-like and macrophage-like cells [68].
Expansion of these cells leads to a hyperplastic synovial lining layer referred to as a “pannus”. The pannus invades the periarticular bone at the cartilage-bone junction and leads to the progressive destruction of cartilage and subchondral bone tissue [69].
Matrix metalloproteinases are crucial for the pathogenesis of RA. Synovial fibroblast-like cells, having a tumor-like appearance, secrete various proteases, including MMPs that degrade ECM components, mainly proteoglycans, and collagens, of articular cartilage in the affected joints. It is in contrast to OA, where chondrocytes of affected joints are the primary cellular source for destructive proteinases. Expression of the following MMPs is upregulated in synovial tissue: MMP-1, -3, -9, and -13 [12–14].
Naringin and taraxasterol – potential treatment in rheumatoid arthritis
In a study from 2021, therapeutic effects and inherent mechanisms of naringin, a flavanone glycoside of naringenin, against RA were elucidated via network pharmacology and in vitro experimental validation.
It has been reported both in vitro and in vivo that naringin decreases the production of inflammatory mediators and proinflammatory cytokines and attenuates clinical symptom severity [70, 71].
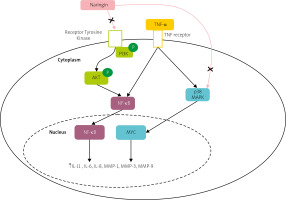
However, the therapeutic effect and inherent mechanisms of naringin in human RA fibroblast-like synoviocytes (RA-FLS) remained ambiguous. A study demonstrated that naringin inhibits the mRNA expression of inflammatory cytokines, and MMPs, including MMP-1, MMP-3, MMP-9, and MMP-13, and promotes apoptosis in RA-FLS via PI3K/Akt and MAPK/ERK signaling pathways, as shown in Figure 2 [72].
Fig. 2
Potential pathway of naringin effect on the expression of matrix metalloproteinases and proinflammatory cytokines in TNF-α induced RA-FLS. Naringin inhibits both the PI3K/Akt and MAPK/ERK signaling pathways.
AKT – protein kinase B, NF-κB – nuclear factor kappa-light-chain-enhancer of activated B cells, P – phosphate, PI3K – phosphatidylinositol 3-kinase, p38 MAPK – p38 mitogen-activated protein kinase.
Looking at these results one can speculate that naringin alleviates articular cartilage and bone erosion in RA by inhibiting synovial tissue secretion of MMPs and thus may act as a natural anti-inflammatory agent in RA.
A different plant-derived substance that according to recent studies has therapeutic potential in RA is taraxasterol (TAR), one of the bioactive pentacyclic triterpenes isolated from Taraxacum officinale. Taraxasterol significantly suppressed the expression of pro-inflammatory cytokines, and the production of MMPs, e.g. MMP-1 and MMP-3, in both human fibroblast-like synoviocytes rheumatoid arthritis (HFLS-RA) in vitro and collagen-induced arthritis (CIA) mice in vivo [73].
In conclusion, current findings suggested that TAR might be one of the considerable therapeutic compounds to attenuate RA development.
Coenzyme Q10 and Stachys schtschegleevii supplementation in rheumatoid arthritis
A randomized, double-blind, placebo-controlled trial assessed the effect of coenzyme Q10 (CoQ10) supplementation on serum MMPs and clinical parameters in RA patients, and it seems that CoQ10 may provide a new complementary treatment for patients with RA, as it affected MMP-3 serum levels and decreased DAS28 (Disease Activity Score) in patients [74].
Another clinical trial (triple-blind, randomized controlled clinical trial) focused on the effects of Stachys schtschegleevii (SCC) tea on women diagnosed with moderately active RA. The SSC administration led to a reduction in the number of DAS28, and serum levels of MMP-3 [75].
Nonetheless, this treatment still needs additional research, as the active metabolites causing levels of MMPs to drop were not identified. All the major possible MMP-targeting treatments for OA and RA mentioned in this review are shown in Table I.
Table I
The major possible MMP targeting treatments for osteoarthritis and rheumatoid arthritis
| Osteoarthritis | Rheumatoid arthritis | ||
|---|---|---|---|
| Reference | Treatment | Reference | Treatment |
| Favero et al. (2019) [23] | TIMPs employment | Aihaiti et al. (2021) [72] | Naringin administration |
| Zeng et al. (2019) [26] | FOXM1 knockdown | Chen et al. (2019) [73] | Taraxasterol administration |
| Yang et al. (2018) [27] | IRF-8 knockdown | Nachvak et al. (2019) [74] | CoQ10 supplementation |
| Jiang et al.(2017) [28] | BRD4 inhibition by JQ1 | Mirtaheri et al. (2022) [75] | SCC tea consumption |
| Qu et al. (2018) [43] | Ghrelin administration | Liu et al. (2019) [77] | PARP2 inhibition by miRNA-125 |
| Inagaki et al. (2017) [44] | 3-B2 in combination with low-dose betamethasone | ||
| Gao et al. (2020) [47] | Intra-articular injection of hyaluronic acid with iron-glutathione complex | ||
| An et al. (2020) [51] Li et al. (2019) [52] Bai et al. (2019) [53] | miRNA knockdown – miRNA-203a, miRNA-103, miRNA-122 | ||
| Lei et al. (2019) [55] Zhao et al. (2018) [56] | lnRNA – miRNA-16-5p suppression via SNHG1, PVT1 suppression | ||
[i] BRD4 – bromodomain-containing protein 4, 3-B2 – 2-(8-methoxy-2-methyl-4-oxoquinolin-1(4H)-yl)-N-(3-methoxyphenyl) acetamide, 10FOXM1 – forkhead box M1, CoQ10 coenzyme – Q10, IRF-8 – interferon regulatory factor-8, PARP2 – poly(ADP-ribose) polymerase 2, PVT1 – plasmacytoma variant translocation 1, SCC – Stachys schtschegleevii, SNHG1 – small nucleolar RNA host gene 1, TIMPs – tissue inhibitors of metalloproteinases.
MicroRNAs in rheumatoid arthritis
MicroRNAs are a family of small non-coding RNAs that are involved in the occurrence and development of RA [76]. It was found that miRNA-125 was downregulated and poly(ADP-ribose) polymerase 2 (PARP2) was upregulated in synovial cells taken from RA-affected rats. Synovial cells cotransfected with miRNA-125 mimics, and PARP2-siRNA had significantly lower expression of pro-inflammatory cytokines, MMP-1, p-PI3K, p-Akt, and p-mTOR in comparison with the negative groups.
Poly(ADP-ribose) polymerase 2 was directly inhibited by miRNA-125. The authors concluded that miRNA-125 might relieve RA progression through regulation of the PI3K/Akt/mTOR signaling pathway via directly inhibiting PARP2 expression [77].
However, there is still one major obstacle that must be overcome before miRNA-based therapy is approved for clinical trials: drug administration. No efficient delivery system that is minimally invasive has been found. Currently the preferred delivery systems are the parenteral or local route, as miRNAs have reduced intestinal absorption via the oral route [78].
In the case of miRNA therapies in RA, local delivery (intraarticular) might be beneficial, because a greater amount of active substance might reach the site of RA progression and possible systemic effects of miRNAs could be reduced. Since one miRNA targets several genes, adverse effects are possible. In the past two clinical trials of miRNAs had to be discontinued, due to adverse effects suffered by patients: immune-related adverse events and hyperbilirubinemia [79].
Lipid-based vesicles are used for agent delivery, because they are able to cross the cell plasmatic membrane and have low cytotoxicity, but there are other more effective delivery systems available, such as nanoparticles, e.g. exosomes [80].
Exosomes are nanosized (40–100 nm) extracellular vesicles found in mammals that promote intracellular signaling by transporting lipids, proteins, and genetic material such as miRNAs. Exosomes are produced by many types of cells, e.g. reticulocytes, epithelial cells, neurons, and tumor cells [81].
They do not elicit an immune response, and can be isolated from mesenchymal stem cells (MSCs). Mesenchymal stem cell-derived exosomes have been reported to improve osteochondral regeneration and downregulate angiogenesis and MMP-14 in RA FLS [82, 83].
Compared to conventional lipo-based vesicles and manufactured nanoparticles, exosomes are more biocompatible and readily absorbed by target cells due to their membrane proteins and endogenous origin. Exosomes are more stable in bodily fluids than similarly structured liposomes [84]. Exosomes present the possibility to become delivery agents for therapeutic molecules.
To date, two miRNA drugs have completed phase II clinical trials (NCT02826525 and NCT03601052) [85, 86]. However, in neither case has recruitment for phase III begun.
Association between anti-citrullinated protein antibodies and matrix metalloproteinase
Anti-citrullinated protein antibodies (ACPAs) and rheumatoid factor (RF) are autoantibodies detectable in 70–80% of RA patients. Anti-citrullinated protein antibodies are highly specific for RA, while RF can also be present in other diseases. About 1/3 of RA patients are ACPA or RF negative, which makes early diagnosis of RA challenging.
A study showed that MMP-3 levels in ACPA-positive and ACPA-negative RA patients were significantly higher than in healthy models. The study highlighted the positive correlation between MMP-3 serum levels and disease activity/inflammatory markers. Matrix metalloproteinases-3 may be used as a potential marker for early diagnosis of ACPA-RA [87].
A different study identified a significant correlation between ACPA and MMP-9 levels in synovial fluid compared to RF and MMP-9 level [88]. This highlighted the importance of the ACPA parameter over RF for diagnosis of early RA.
On the other hand a different study found that elevated MMP-3 levels, among other markers, at disease onset were associated with achieving sustained disease-modifying antirheumatic drug-free remission in ACPA-negative patients. MMP-3 might help identify patients with a high likelihood of achieving sustained disease-modifying antirheumatic drug-free remission [89].
Conclusions
This review summarizes the mechanisms responsible for degenerative progression involved in OA and RA, specifically the role of MMPs in them. Both OA and RA target joints and can lead to their progressive disability, which is a burden not only on affected individuals but also on the healthcare system.
Analyzing the molecular background of degenerative and inflammatory arthritis such as inflammatory mediators, and, inhibitors creates a potential for developing novel targeted methods of treatment as well as biomarkers indicating the level of disease progression.
In addition, genetic molecular analysis can lead not only to possible therapeutic targets but also to the susceptibility of specific populations to diseases. Despite the promising findings outlined in this review, MMP-targeted research is still in the very early stages, and it does not seem possible that any new groundbreaking therapies targeting MMPs will appear in the near future.