Introduction
Polymyositis (PM) and dermatomyositis (DM) are rare idiopathic inflammatory myopathies (IIM) characterized clinically by symmetrical proximal muscle weakness due to inflammatory cell infiltrates in skeletal muscles, and the involvement of endomysial layers of skeletal muscles in PM or perimysial layers of muscles in DM [1].
The prevalence of PM/DM is approximately 2–22 per 100,000 individuals worldwide [2–7]. Polymyositis rarely presents in childhood and usually affects people above the age of 20 years. Dermatomyositis, however, has a bimodal age distribution affecting the population between 5 to 15 years and 45 to 60 years [2].
The most commonly used criteria for PM and DM are Peter/Bohan criteria which include symmetric proximal muscle weakness, elevated serum muscle enzymes, myopathic changes in electromyography (EMG), characteristic muscle biopsy abnormalities, and the typical rash of dermatomyositis. Apart from skeletal muscles involvement, the skin and internal organs may be affected as well.
The first case of myocardial involvement in patients with polymyositis was described by Oppenheim in 1899 [8]. Due to the lack of strict criteria for defining IIM-related cardiac injury and the different diagnostic approaches used to assess it, there are wide discrepancies regarding the estimated incidence of myocardial involvement in polymyositis.
According to the EuroMyositis registry, clinically overt myocardial involvement, defined as the presence of pericarditis, myocarditis, arrhythmia, or sinus tachycardia in the course of PM/DM, was found in 9% of patients [9].
Dankó et al. [10] observed symptoms of heart failure or myocarditis and/or abnormalities in imaging tests (electrocardiography – ECG, echocardiography) in 9.3% of patients with PM and 4.7% of patients with DM, after excluding other causes of the above changes. A higher percentage of abnormalities in the ECG and echocardiography was observed in the research of Spanish authors [11] – 27% among patients with PM and 30% of patients with DM, respectively.
More often used modern diagnostic methods allow for to detection of features of subclinical damage of the myocardium. The incidence rate could reach 64% [12]. Infiltration of mononuclear inflammatory cells, focal myocyte necrosis, and fibrosis are described in autopsies. The changes primarily were located interstitially and perivascularly [13]. There is also evidence of small vessel involvement (media hyperplasia) similar to skeletal muscle [13, 14].
Myocardial involvement in patients with inflammatory myopathies is an unfavourable prognostic factor and one of the most common causes of mortality in this group of patients [10, 11, 15]. Dankó et al. [10] found that cardiovascular deaths most often occur after an average of 5 years of the main disease course.
Among 160 patients with PM/DM, 22% of deaths were due to cardiovascular causes [16]. Thus, screening for myocardial damage in patients with PM/DM seems to be an important part of clinical practice.
The authors present a review of the literature covering the issue of cardiovascular manifestations observed in IIM with special emphasis on diagnostic issues. Eighty-two articles from 1971 to 2021 were retrieved from the literature search. Current knowledge about the role of blood assays (especially troponin), ECG, echocardiography and cardiac magnetic resonance (CMR) in the diagnostic process has been summarized. Finally, we propose a strategy for early detection of heart involvement in IIM.
Material and methods
We searched via PubMed potentially relevant articles from the literature published between 1971 and 2021. To identify relevant publications we applied the following inclusion criteria: publication language: English; type of study: controlled trials, observational studies, qualitative studies, case descriptions; participants/populations: humans, adult aged 18 years or over; key words: polymyositis OR dermatomyositis, AND heart OR heart diseases OR myocardium OR myocarditis OR cardiomyopathy OR heart failure.
A thorough review of the area of interest was then manually conducted to search for articles that presented actual data. The bibliographies of the retrieved articles were manually searched to identify additional articles and other documents. Finally, the authors included in the analysis and discussion 82 articles. A diagram of the search design is shown in Figure 1.
Results and discussion
Symptoms of suggestive myocardial involvement in polymyositis/dermatomyositis
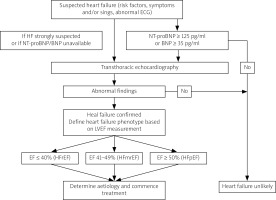
Symptoms of heart failure (HF) are most frequently reported in the population of patients with PM/DM, of which exertional dyspnea is found in 11–57% of patients [12, 17, 18], and orthopnea in 12% of patients [17]. However, these symptoms could be the result of respiratory muscle weakness or lung involvement in the course of the underlying disease, and therefore require a differential diagnosis. The algorithm for diagnosing HF according to the European Society of Cardiology (ESC) guidelines is shown in Figure 2.
Fig. 2
Algorithm for diagnosing HF according to the European Society of Cardiology guidelines.
BNP – B-type natriuretic peptide, ECG – electrocardiogram, HF – heart failure, HFmrEF – heart failure with mildly reduced ejection fraction, HfpEF – heart failure with preserved ejection fraction, HFrEF – heart failure with reduced ejection fraction, LVEF – left ventricular ejection fraction, NT-proBNP – N-terminal pro-B type natriuretic peptide.
During clinical evaluation special focus should be given to patients with previously diagnosed heart disease – in this group systemic inflammation in the course of PM/DM may cause exacerbation of HF. On the other hand, the occurrence of new symptoms in patients with no previous history of heart disease may suggest myocarditis. Clinically justified suspicion of myocarditis is based on the coexistence of symptoms:
dyspnoea, chest pain, often of a pericardial nature, palpitations,
biochemical features of myocardial damage,
and/or abnormalities in imaging tests, e.g.: ECG, echocardiography, magnetic resonance imaging (MRI), with the absence of significant stenoses in the coronary arteries, pre-existing heart damage (severe valvular disease, known cardiomyopathy) or non-cardiac causes of such abnormalities, e.g. hyperthyroidism [19].
In an autopsy study involving 20 patients with PM, 6 patients (30%) had histological features of myocarditis, of which 4 had symptoms of HF previously [13].
Linos et al. [20] reported that only 48% of patients with MRI-confirmed myocarditis noted symptoms related to cardiac involvement. Cases of patients requiring myocardial transplantation due to severe HF in the course of myocarditis in DM have been reported rarely [21]. So far, no relationship between PM/DM activity and myocardial involvement has been found.
Chest pain was reported by 4% of patients with PM/DM [17]. The risk of developing coronary artery disease in this group of patients was estimated to be 3 times higher than in the general population. A higher incidence rate of cardiovascular events has been demonstrated in patients with inflammatory myopathies [22–24].
Rai et al. [22] concluded that the incidence of myocardial infarction was significantly higher in the population of patients with PM/DM compared to the control group, especially during the first year after the diagnosis of PM. The coexistence of DM and heart disease significantly worsened the prognosis in comparison with patients diagnosed only with DM or only with cardiovascular disease [22].
Several aspects contribute to the higher risk of atherosclerosis in inflammatory myopathies. Firstly, the relationship between generalized inflammation and the development of atherosclerosis and the occurrence of cardiovascular diseases developing on its basis has been documented [25]. Secondly, patients with PM/DM have a higher prevalence of risk factors for coronary artery disease.
Diederichsen et al. [12] reported, that hypertension was present in 72% of patients vs. 42% in the control group and type 2 diabetes – in 13% and 0% of patients, respectively. Higher levels of triglycerides have also been reported among patients with PM/DM [12]. Thirdly, chronic glucocorticosteroid therapy used in the PM/DM group increased the risk of developing arterial hypertension and diabetes type 2 diabetes [26]. Active search and correction of risk factors for coronary artery disease in this group of patients is an important element of clinical practice.
Palpitations were reported by up to 34% of patients with PM/DM [17, 27]. It has been shown that the incidence of supraventricular arrhythmias is higher in the above group of patients than in the control group [28]. According to Beak et al. [27], atrial fibrillation was observed in 3.5% of patients with inflammatory myopathies (without dividing into groups depending on the etiology of IIM), which were statistically significantly more often than in patients with other autoimmune rheumatic diseases.
There are cases of patients in whom symptomatic ventricular arrhythmias were the first symptom of heart damage in the course of DM/PM [29–31]. Cardiac arrhythmias are provoked by an active inflammatory process in the myocardium, as well as myocardial fibrosis as a result of chronic inflammation.
Syncope – single rare cases of syncope due to complete atrioventricular block were described as the first manifestation of PM [32].
Diagnostic approaches
Apart from evaluation of clinical cardiac status with assessment of symptoms and traditional cardiovascular risk factors, cardiac biomarkers and cardiac imaging may help improve the diagnostic strategy.
Troponins
Troponins are part of the contractile apparatus of heart-striated muscle tissue. Cardiac troponins: I (cTnI) and T (cTnT), considered to be specific for the heart muscle, are biomarkers used for years in the diagnosis of myocardial infarction according to the algorithm from the ESC guidelines [33]. It is known that elevation of the level of cardiac troponin may occur in situations others than acute myocardial infarction (Table I).
Table I
Reasons for elevated troponins because of myocardial injury
Detection of increased blood cTn concentration above the 99th percentile reference threshold (URL) could be defined as heart injury. According to the ESC recommendations, both cTnI and cTnT, especially their highly sensitive forms, can be used to detect myocardial injury as specific markers of myocardial cell necrosis [33].
Meanwhile, in patients with IIM, the situation turns out to be more complex. Initially, troponin T was mainly used to assess myocardial damage in patients with inflammatory myopathies [34].
However, this strategy was challenged in subsequent studies comparing cTnT and cTnI levels in people with active IIM. Erlacher et al. [33] observed an increase in cTnT in 41% of patients with PM/DM, which correlated with other markers of muscle damage (increase in creatine kinase – MB in 51% of patients) and the results of tests assessing disease activity. No electrocardiogram or echocardiogram abnormalities were found in any of the patients [35].
Aggarwal et al. [34] found no association with abnormal cTnT results with myocardial involvement on the basis of ECG and echocardiography: 78% of patients with IIM had elevated cTnT levels. On the contrary, abnormal levels of cTnI were rarely found in patients with IIM, while abnormal levels correlate with myocardial involvement observed in imaging studies [12, 27, 35, 36].
An analysis of 123 patients with IIM [37] (30% with DM and 28% with PM) showed high specificity and positive predictive value for elevated cTnI and myocardial involvement, which was found in 62% of patients with abnormal TnI compared to only 23% of patients with elevated creatine kinase (CK) and 21% of patients with abnormal cTnT.
Troponin T level correlated with the global assessment of disease activity more than the cTnI and CK levels. High sensitivity (87%) and specificity (84%) of elevated troponin I level for myocardial involvement have been demonstrated in patients with MRI-confirmed active myocarditis in the course of IIMs [20].
The advantage of cTnI as a biomarker of myocardial damage is related to its expression only in myocardial cells, while increased cTnT concentration has been described, among other situations, in developing foetuses and during regeneration of damaged skeletal muscles in adults [38–41].
Natriuretic peptides
Assessment of plasma natriuretic peptide (NP) concentrations is recommended as an initial diagnostic test in patients with suspected heart failure (HF). It has diagnostic and prognostic significance. The diagnosis of HF in people with normal NP values is unlikely [42].
Few studies have assessed the concentration of NP in patients with IIM. Among patients with PM/DM without clinical signs of myocardial involvement and normal left ventricular ejection fraction, elevated N-terminal pro B-type natriuretic peptide (NT-pro BNP) levels were found in 32% of patients [43].
The level of NT-pro BNP correlated with features of diffuse myocardial fibrosis in T1-weighted images in magnetic resonance imaging, regardless of the duration of PM/DM [43]. High sensitivity (95%) and specificity (93%) of abnormal NT pro-BNP concentration for the detection of myocardial involvement have been demonstrated.
In patients with myocarditis confirmed in the CMR study, the concentration of NT-pro BNP was statistically significantly higher compared to the control group (mean concentration of NT-pro BNP 2028 pg/l vs. 82 pg/l respectively, p < 0.001) [20].
Antibodies
Among the serological tests used in the diagnosis of IIM, an important position is occupied by the assessment of the presence of myositis specific antibodies (MSA) and antibodies associated with myositis (MAA). In a study of 55 patients with PM [44], the presence of anti-Ro antibodies was found in 69% of patients with myocardial involvement: the main clinical manifestation was complete atrioventricular block.
There is a relationship between the presence of antimitochondrial antibodies type M2 (AMA-M2) and a more severe course of cardiac involvement in the course of IIM. Antimitochondrial antibodies type M2 were used primarily in the diagnosis of primary biliary cirrhosis (PBC).
They have also been found in other autoimmune diseases, including inflammatory myopathies. It is estimated that AMA-M2 antibodies are present in 5–11.3% of patients with IIM [45, 46]. Previous studies indicate a correlation between the presence of AMA-M2 and myocardial involvement in patients with IIM [20, 45, 47].
Electrocardiography
Electrocardiography is the basic method of evaluating arrhythmias and conduction disorders. Among patients with PM/DM, abnormal ECG recordings were found in 32–85% of patients [17, 48]. However, these changes are non-specific. Of these, ST-T segment changes (58%), premature ventricular beats, left anterior bundle branch block (4–13%), and right bundle branch block (4–9%) are the most common [17, 48].
No relationship was found between these disorders and the clinical activity of PM or the duration of the disease [48]. In autopsy studies of patients with PM/DM with conduction disorders, it was found that inflammatory infiltrates and fibrosis were most intense within the conduction system of the heart [49].
In studies comparing the ECG records of patients with PM/DM to a healthy control group, changes were observed in 28.6–43% patients vs. 8.1–14% respectively [12, 50, 51], of which statistical significance was demonstrated only for the longer QTc interval (p = 0.0001), longer duration of QRS complexes (p = 0.007) and signs of left ventricular hypertrophy in the PM/DM group (10.7 vs. 1.2%, p = 0.008) [27, 50, 51].
Comparing patients with PM and DM, ECG abnormalities e.g.: features of left ventricular hypertrophy, left atrial enlargement, supraventricular and ventricular arrhythmias, left anterior bundle branch block, were found more frequently in patients with PM (p < 0.05) [50].
In the Holter ECG study, a higher mean heart rate and a higher number of supraventricular arrhythmias are found in the group of patients with PM/DM compared to the control group [12, 27].
Echocardiography
Echocardiography is a widely available tool for assessing cardiac function. In the group of patients with IIM, features of left ventricular diastolic dysfunction (LVDD) are most common (34.5–42%) [51, 52]. In a study comparing patients with PM/DM with a control group [53], a statistically significantly higher incidence of LVDD was found in the study group, which correlates with the age of the patients and the duration of the disease.
Péter et al. [54] assessed, parameters of diastolic function in 30 patients with newly diagnosed PM/DM at baseline and 3 months after starting steroid therapy. In the initial examination, tissue Doppler parameters (E/A, E/E’) were normal, but after 3 months of therapy, features of first degree diastolic dysfunction were found, which may indicate progressive subclinical myocardial involvement in the course of the underlying disease.
Left ventricular systolic dysfunction, defined as a decrease in left ventricular ejection fraction, is rarely observed in the PM/DM group without pre-existing heart disease. Most studies showed no difference between LVEF in the PM/DM group and the control group [51, 52, 54, 55].
The use of modern imaging techniques significantly improves the sensitivity in detecting subclinical myocardial contractile dysfunction. Studies using the assessment of global longitudinal strain (GLS) in patients with PM/DM showed its statistically significant reduction in both the left (LVLS) and right ventricle (RVLS) compared to the control group (LVLS 47% vs. 3%, RVLS 58% vs. 7%, respectively [50, 56].
In the group of patients with PM/DM diagnosed for > 1 year, an independent correlation between reduced LVLS and myositis damage index (MDI) was found, while reduced RVLS correlated with the presence of interstitial features lung involvement [52].
In older studies, enlargement of the left atrial and left ventricular cavities (8–15% of patients) and thickening of the left ventricular muscle in patients with PM/DM (8–15%) were described [52]. However, the data were not correlated with age, gender, and comorbidities affecting structural changes in the heart.
In more recent studies, enlargement of the left atrial and left ventricular cavities in patients with PM/DM compared to the control group (71% vs. 12.9% and 48% vs. 0%, respectively) was found in patients with features of myocarditis (in cardiac magnetic resonance imaging). In these patients, regional (25.8% vs. 0%) and generalized (48.4% vs. 0%) disturbances of the left ventricular systolic function are more often observed [36].
Pericarditis, pericardial effusion, and tamponade are rare in patients with IIM. However, there are cases of patients with PM/DM in whom tamponade was the first symptom of myocardial involvement [57, 58].
Routine echocardiography should be considered as part of the evaluation of patients with PM/DM, especially when myocardial involvement is suspected [59].
Cardiac magnetic resonance
Cardiac magnetic resonance (CMR) is a non-invasive diagnostic method used in cardiology to assess the morphology and function of heart structures, as well as its perfusion and vitality. According to the guidelines of European and American societies [59, 60], it is used to differentiate the causes of cardiomyopathy (ischemic vs. non-ischemic).
Suspicion of myocarditis is a challenging diagnosis due to non-specific clinical presentation and diverse etiology. The most common cause of myocarditis is viral infections, but inflammatory changes are also observed in the heart in the course of systemic diseases, including inflammatory myopathy.
Cardiac MR allows for unique tissue imaging, especially of the extracellular space, which gives the possibility to assess elements characteristic for inflammation (edema, hyperaemia, capillary leak, necrosis, and finally fibrosis) independently of the etiological factor. However, due to the natural evolution of changes in the course of myocarditis accurate detection is obtained when CMR is present in the first few days from the onset of the disease [61–63].
Limitations of classical CMR imaging methods may underestimate the detection of inflammatory lesions involving a large part of the heart muscle (no reference tissue). Skeletal muscle is the reference in these situations, but in the case of concomitant skeletal muscle disease (as in IIM) there is still a risk of false-negative results. The introduction of new mapping techniques to the clinic with the quantification of T1 and T2 relaxation times improves diagnostic capabilities.
According to the consensus published in 2020 by the Society of Cardiovascular Magnetic Resonance (SCMR) [64] in patients with suspected myocarditis, the updated Lake Louise criteria assessing myocardial oedema (in T2-weighted images) and features of myocardial damage in T1 sequences should be used in the CMR examination: enhanced signal in T1 sequences, increased volume of extracellular space and late post-contrast enhancement with distribution characteristic of non-ischemic cardiomyopathy, indicating the presence of myocardial fibrosis.
In myocarditis, late gadolinium enhancement (LGE) foci are typically located intramuscularly or subepicardially, and their location often suggests a specific etiological factor. Additional criteria include the presence of features of pericarditis and left ventricular systolic dysfunction [63].
The features of myocarditis in MRI are observed in 50–62% of patients with idiopathic inflammatory diseases of the muscles [65, 66]. Rosenbohm et al. [65] found the presence of LGE in 65% of patients with PM and 54% of patients with DM (most of them under pharmacological treatment for IIM), with LGE localization predominating in the lateral and lower segments of the left ventricle. The presence of LGE was statistically significantly correlated with reduced ejection fraction observed in 9% of patients. However, there was no correlation between myocarditis features in CMR and clinical symptoms [65].
New techniques using mapping sequences and assessing the features of subclinical left ventricular dysfunction (global deformation, strain) in patients with IIM bypass the limitations of classical techniques in detecting disseminated inflammatory lesions and potentially detect features of myocardial involvement at earlier stages of the disease.
In the work of Xu et al. [67] from 2020, 44 patients with newly diagnosed IIM were compared with a 30-person control group. Among patients with IIM statistically significantly prolonged T1 and T2 relaxation times and extracellular volume (ECV) were found. Late gadolinium enhancement was described in 23% of patients.
In the one-year follow-up (after IIM treatment) a statistically significant reduction of T1 T2 and ECV was observed, while LGE did not change, which proves the irreversibility of myocardial fibrosis found in CMR [67].
In another study [68] prolonged T1 global relaxation time and increased ECV were found in patients with IIM who had not yet observed LGE or left ventricular systolic dysfunction compared to healthy controls.
According to the authors, the T1 mapping technique may be a sensitive method for detecting subclinical cardiac involvement in patients with IIM. In the analysis of the global deformation of the left ventricular muscle, no statistically significant differences were found between the study group and the control group [68].
In asymptomatic patients with PM/DM, the presence of LGE was found significantly more often than in the healthy control group (response 19% vs. 0%) [42]. In the study group there were also higher values of T1 and ECV times, which significantly correlated with the concentration of NT pro BNP [42].
Among patients with PM, ECV values were higher compared to patients with DM (33% vs. 30%, respectively), and the percentage of left ventricular muscle segments with abnormalities was higher [69].
It is known that in the acute phase of myocarditis, death due to heart failure and/or arrhythmias could occur [70, 71]. Some patients with IIMS also develop chronic HF, which is an unfavorable prognostic factor.
Calcifications in the coronary arteries (calcium score)
Diederichsen et al. [72] assessed the incidence of classic risk factors and coronary calcifications in a group of 76 patients with PM/DM compared to a healthy control group. In the study group, a high calcium score (> 400) was found significantly more often, respectively in 20% of patients with PM/DM and 4% in the control group (p = 0.04).
However, the multivariate analysis showed no relationship between the calcium score and the presence of PM/DM. An elevated calcium score correlated with age and smoking.
Algorithm of the procedure
So far, there are no clear guidelines for the diagnosis of myocardial involvement in the course of inflammatory myopathies. There are many discrepancies in the available studies regarding the definition of myocardial involvement. A large range in the incidence of changes in the cardiovascular system results from the differences in the studied groups of patients and the use of different diagnostic methods.
Older studies used clinical symptoms, ECG changes, and echocardiography. Assessment of diastolic function was often based on outdated recommendations of the European Society of Echocardiography. New studies using magnetic resonance imaging showed features of subclinical myocardial involvement in a large group of asymptomatic patients.
The prognostic significance of the abnormalities found is not clear. In one retrospective analysis, patients with baseline elevated troponin levels (82% troponin T and 2.5% troponin I) without clinical evidence of cardiac involvement did not develop symptoms during an average of 24.5 months of follow-up [34].
The prognostic value of reduced GLS has been found in some cardiac conditions (including hypertrophic cardiomyopathy, acute coronary syndrome) [73–75]. Further prognostic evaluation is required in patients with IIM. Similarly, in the case of CMR, the relationship between LGE and worse prognosis, e.g. in hypertrophic or dilated cardiomyopathy, has been described [76, 77]. In the case of patients with IIM, however, the prognostic significance of the above-mentioned changes is not clear and requires further research.
In symptomatic patients (dyspnea on exertion, chest pain, palpitations, fainting), diagnostics should be performed according to the recommendations of the European Society of Cardiology, with particular emphasis on myocarditis/acute coronary syndrome [78, 79].
According to Chen et al. [80] troponin I (preferably high-sensitivity tests), ECG and echocardiographic assessment are proposed in all patients with IIM. In the case of abnormalities in the above-mentioned tests, a comprehensive cardiac evaluation should be made in terms of other causes of the changes found. Only after such an assessment should CMR be considered.
If the troponin I concentration is below the upper reference limit and there are no abnormalities on the ECG and echo, according to the authors, myocardial involvement is unlikely. If the troponin I result is negative (high-sensitivity tests), but ECG/echo abnormalities are found, first of all, other causes of the above-mentioned changes should be excluded [80].
The recommendation for initial echocardiographic assessment in asymptomatic patients with known systemic disease that may be related to structural heart disease is also included in the guidelines of American societies (American College of Cardiology Foundation, American Society of Echocardiography, ACCF/ASE/AHA, etc.) [59].
Recommendations regarding cardiac magnetic resonance imaging in patients with connective tissue diseases (CTDs) are included in the consensus of the International Consensus Group on CMR in Rheumatology. They include the following [81]:
to evaluate patients with acute or chronic typical/atypical cardiovascular symptoms and normal results of routine non-invasive diagnostics,
to assess the possibility of asymptomatic myocarditis in patients with inflammatory myopathies and normal results of routine non-invasive diagnostics,
in the assessment of patients with CTDs and acute left ventricular dysfunction or new right or left bundle branch block, atrioventricular block or arrhythmias regardless of the results of other tests,
in patients in whom the results of non-invasive diagnostics are ambiguous or do not explain the symptoms presented by the patients.
However, the authors emphasize the need to conduct research on a larger group of patients and the need to assess the prognostic significance of CMR changes.
Some reports suggest a beneficial effect of immunosuppressive therapy on changes in the myocardium. Allanore et al. [82] reported a series of 4 patients with confirmed myocarditis in IIM (2 with PM, 1 with DM, 1 with PM and concomitant systemic sclerosis) treated with methylprednisolone pulses followed by immunosuppressive therapy. After 2 months of follow-up, the clinical symptoms related to the involvement of the heart muscles disappeared, and after 6 months, the post-contrast enhancement in the follow-up CMR was reduced.
In another study [54] after 3 months of steroid therapy, normalization of subclinical systolic dysfunction in echocardiography was observed (tissue Doppler – TDI and mitral annular plane systolic excursion – MAPSE).
On the other hand, Xu et al. [67] observed no changes in the LGE areas found at baseline and in the 12-month follow-up after starting IIM treatment, which may suggest irreversibility of the changes.
Conclusions
Initial cardiac evaluation is recommended in all IIM patients. Diagnostic steps in assessment of cardiac involvement should include full clinical history, signs/symptoms of cardiac disease, baseline ECG and echocardiography. Cardiac troponin I is the preferred marker of myocardial injury in patients with IIM.
Correlations between other biomarkers and myocardial involvement in patients with IIM require further research. Cardiac MR is a useful tool in diagnosis of myocardial inflammation in IIM and early detection of subclinical cardiac involvement.
However, the need for comprehensive cardiac evaluation in asymptomatic PM/DM patients and the prognostic value of subclinical cardiac changes in this group of patients have to be evaluated in prospective studies.