Introduction
Rheumatic diseases in children are relatively rare, with a prevalence of about 10–14 cases per 100,000 in the developmental age population – data from 2014 [1].
The imaging examinations most commonly used in the diagnosis of rheumatic diseases in children are joint ultrasonography (US), classical radiological assessment (X-ray), and the increasingly used musculoskeletal magnetic resonance imaging (MRI). However, it should be noted that establishing an accurate diagnosis is possible only through a detailed analysis of the clinical presentation, the course of the disease, a medical interview and the results of laboratory and imaging tests. A histopathological examination may prove crucial to establish a definitive diagnosis. Early diagnosis of rheumatic disease in children and adolescents is important because of the increasing possibility of applying an appropriate form of comprehensive treatment, including targeted pharmacotherapy that offers the possibility of permanent remission. This is particularly relevant in children, as delaying treatment of rheumatic disease can lead to growth and developmental disorders and permanent disability [2].
Magnetic resonance imaging of the musculoskeletal system is a common tool in the diagnosis of rheumatic diseases, and in some it is a part of the diagnostic criteria, for instance, in inflammatory myopathies [3].
This imaging method is of primary importance in the diagnosis of juvenile idiopathic arthritis (JIA), chronic non-bacterial osteomyelitis (CNO) and juvenile idiopathic inflammatory myopathies (JIIMs), and of great value in the diagnosis and monitoring of all other rheumatic diseases.
A major advantage of MRI examination is that it does not involve any ionizing radiation, and is therefore completely safe. Moreover, it is widely available, and the introduction of modern technological solutions and advanced software has significantly reduced the time of a single examination. This has made it possible to use MRI as a tool to look for inflammatory lesions within the whole body (WB-MRI), and thus to assess the patient’s condition most accurately [4].
Magnetic resonance imaging protocol in the diagnosis of rheumatic diseases
Magnetic resonance imaging protocols should be adjusted considering the specifications of the scanner, available coils and image quality for each sequence [5]. The recommended maximum section thickness is 3 mm with an intersection gap of 0.3 mm, depending on the anatomical structure being evaluated. Thinner sections should be made for small joints [5], especially within the hand. For large joints (knee, shoulder, hip, ankle), a thickness of 3–4 mm should be used. The following sequences are evaluated: T1-, T2- and PD-weighted with or without fat suppression (FS) or short tau inversion recovery/turbo inversion recovery magnitude (STIR/TIRM) sequences, as well as available post-contrast sequences. These allow the diagnosis of pathological changes in the synovial membrane, subchondral bone marrow and surrounding soft tissues [5, 6].
T2-weighted images show both fat tissue and fluid/edema with high signal intensity. In particular, such images are useful when FS techniques are applied, in which the signal from fat is suppressed [7]. The T2-weighted sequence with FS (T2FS) increases the detection of edema/fluid localized within fat tissue regions, such as bone marrow edema [5].
T1-weighted images are used because of their relatively short imaging time, good presentation of anatomical details and ability to visualize inflamed synovial membrane after intravenous injection of contrast (gadolinium, Gd, post-Gd images). Gd-enhanced tissues have high signal intensity on T1-weighted images, and because Gd uptake depends on tissue vascularization and perfusion, it is relatively easy to identify highly vascularized synovitis [5] or other inflammatory regions. On T1-weighted images with FS after Gd administration (T1FSGd), FS increases the contrast between synovitis and adjacent structures [5].
Post-contrast images in the T1FS sequence are used to increase the specificity of the diagnosis, mainly to distinguish effusion from synovitis or peritenonitis. However, these are not necessary for evaluating erosions or bone marrow edema [8]. Research evaluating the actual necessity of contrast-enhanced MRI for the assessment of peripheral joints has been conducted, given that the use of intravenous Gd is an invasive procedure, increases the total examination time and cost, and may, although rarely, cause adverse effects (it carries a small risk of nephrogenic systemic fibrosis, only in patients with impaired renal function, i.e., glomerular filtration rate < 30 ml/min/1.73 m2) [8, 9]. In addition, some MRI sequences, such as T2FS and STIR, visualize high water content regions as bright regions. Consequently, edematous regions within the inflamed synovial membrane present increased signal intensity in fluid-sensitive sequences.
Stomp et al. [8] reported that omitting the administration of Gd-based contrast agent results in low specificity for synovitis and low sensitivity for peritenonitis, indicating that the administration of Gd contrast remains essential for optimal diagnosis. Another important advantage of applying Gd contrast is the ability to distinguish exudate from inflammation. Given that even a small physiologic amount of fluid can give a false picture of synovitis on T2-weighted and STIR images, which will negatively affect specificity, it is necessary to perform MR imaging after contrast administration [8].
The advantage of MR examination is the ability to simultaneously obtain cross-sections in any plane: coronal, sagittal, transverse and oblique. Depending on the anatomical location of the structure concerned, cross-sections are made in a specially selected oblique plane, which allows the most accurate assessment of lesions in the particular region. For instance, MRI of the sacroiliac joints is performed in the oblique coronal plane, which is located parallel to the line drawn by joining the posterior edges of the first two sacral vertebrae (S1–S2). In contrast, the preferred imaging plane for WB-MRI is the coronal plane due to the shorter examination time and more accurate evaluation of the long bones. However, the coronal plane has some limitations in evaluating the chest, sternum, skull and spine [10]. In addition, it may have lower sensitivity compared to the transverse plane in detecting target lesions. Therefore, it may be necessary to perform additional sequences in the sagittal plane for the spine and feet or in the transverse plane for the thorax and abdomen, depending on clinical indications [4].
According to the recommendations of the Arthritis Subcommittee of the European Society of Musculoskeletal Radiology [11], MRI examination allows:
assessment of peripheral joints for active inflammation in the form of effusion, synovitis, bone marrow edema, as well as subsequent structural changes such as joint surface damage and erosions of the cortical layer of bone,
evaluation of active inflammatory and structural changes in the sacroiliac joints,
assessment of inflammatory and post-inflammatory lesions of the vertebral joints, i.e. assessment of inflammatory activity, aseptic spondylitis, structural changes in the atlanto-axial/atlanto-occipital joint,
assessment of peritenonitis and enthesopathic changes,
confirmation of clinical diagnosis based on imaging characteristics and/or location of lesions,
qualitative, semi-quantitative and quantitative measurements of active inflammation and chronic joint damage.
Limitations of MRI include [12]:
Juvenile idiopathic arthritis
The most common rheumatic disease of childhood is JIA, which is a complex disease syndrome with varied etiopathogenesis and symptomatology as well as a heterogeneous clinical presentation. The prevalence is estimated at about 1 per 1,000 children, while the incidence ranges from 2 to 28 per 100,000 pediatric population – nearly 10 per 100,000 pediatric population in Poland. The criterion for diagnosis is the onset of the disease before the age of 16 and the persistence of clinical symptoms, including arthritis, for at least 6 weeks and the exclusion of another cause of arthritis from the so-called exclusion list. Typical symptoms of JIA include swelling, exudation and restriction of mobility of the large peripheral joints of the lower extremities. Joint involvement is usually asymmetric with associated morning stiffness [13]. Although the diagnosis of JIA is based mainly on clinical criteria, and US imaging as a supplementary method that is non-invasive, repeatable, low-cost and accessible, in some cases other imaging examinations including MRI may prove valuable in differentiating the disease. Of note, MRI of a single joint, multiple joints or the whole body can significantly expedite the diagnosis of JIA and thus the inclusion of targeted treatment. The most typical joints involved in JIA are the knees, wrists, and ankle joints, but the hands, hips, cervical spine, and temporomandibular joints (TMJ) are also sometimes affected [14]. Crucial signs of JIA on MRI include synovitis, tenosynovitis, bursitis, and enthesitis [15]. The superiority of MRI over US in the evaluation of patients with JIA is due to the possibility of the three-dimensional evaluation of the peripheral and axial joints including complex and deep-seated joints. Magnetic resonance imaging allows detection of bone marrow edema (BME) and osteitis, which cannot be assessed on US [16]. Moreover, Kirkhus et al. [17] found significant differences in bone marrow edema, soft tissue edema, synovial features and cartilage appearance in infectious arthritis, post-infectious arthritis and JIA. The most relevant observations related to the diagnosis of JIA were irregular thickness of the synovial membrane and lack of soft tissue edema. Features such as extensive bone marrow edema, soft tissue edema and decreased contrast enhancement in the bone epiphyses were more commonly found in infectious arthritis [16].
The pediatric-specific MRI scoring system has been developed and validated to standardize and facilitate the assessment of disease in MRI of the musculoskeletal system. The Outcome Measures in Rheumatology (OMERACT) MRI in JIA working group initiated the development of a pediatric WB-MRI scoring system for JIA. This scoring system for JIA focused on the assessment of the inflammation in the joints and entheses of the body [18].
The Juvenile Arthritis MRI Scoring (JAMRIS) system has been developed and validated for the evaluation of inflammatory and destructive changes in the knees of JIA patients. It includes assessment of synovial hypertrophy, bone marrow changes, cartilage lesions and bone erosions [19, 20].
Three MRI scoring systems for TMJ [21–23] were compared and found to be sufficiently reliable in the study of Tolend et al. [24]. To reach a consensus in the MRI scoring systems for TMJ, an international, multidisciplinary expert subgroup was formed within the OMERACT and developed the TMJ-specific scoring system (JAMRIS-TMJ), which was then evaluated, with the result of the reliability of JAMRIS-TMJ being moderate to good depending on the presence of specialty and calibration differences [25]. Magnetic resonance imaging of TMJ proved to be superior to US in the management of patients with JIA not only because of the fact that MRI is sensitive to small amounts of effusion and synovitis but also it enables the assessment of BME, which is crucial as it may be the only pathology. Moreover, US of TMJ cannot detect either secondary osteoarthritis or developmental disorders (condyle flattening, mandibular ramus thinning or shortening, abnormalities of the fovea, and articular eminence) or disc morphology, and cannot evaluate its mobility [16].
Magnetic resonance imaging of the cervical spine has proven to be more applicable than X-rays as subclinical involvement is often necessary to prevent structural and irreversible damage as the majority of early lesions are reversible. In patients with JIA even early ankylosis may develop [26–28]. There are some irreversible lesions which can be detected on MRI as well as on radiography. These are erosions, dens deformations, subluxations, ankylosis, vertebral and disc hypoplasia [28–31]. In the diagnosis of atlantoaxial and subaxial subluxations [32] radiography proves to be superior, while MRI is used to assess neural compression [30, 31]. A scoring system for evaluation of JIA in the spine in the pediatric population is not available.
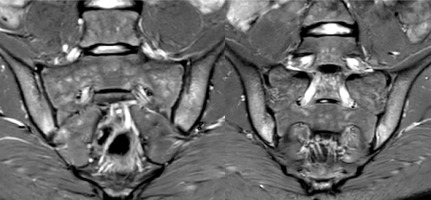
The evaluation of sacroiliac joints (SIJ) is challenging and must be done by radiologists with great experience in pediatric MRI assessment. Children’s immature skeleton developmental appearance may mimic pathological changes in MRI, which can lead to an inaccurate diagnosis [33–36]. For this reason the scoring system for assessing SIJ MRI in adults introduced by the ASAS (Assessment of Spondyloarthritis International Society) [37–39] needed to be adopted for the pediatric population [40]. Several updated recommendations for the definitions of SIJ findings in JIA were developed by the OMERACT Juvenile Idiopathic Arthritis MRI Working Group [41, 42] followed by publication of an atlas of MRI findings of juvenile sacroiliitis to illustrate the updated preliminary OMERACT pediatric JAMRIS scoring system for active and structural lesions [40]. Figure 1 shows MRI of sacroiliac joints with sacroiliitis.
Fig. 1
Two consecutive MRI cross-sections of the sacroiliac joints in STIR sequence showing bilateral bone marrow edema (BME).
The study of Müller et al. [43] highlights the similar problem in evaluation of MRI of the wrist in JIA. Using adult scoring systems and standard MRI sequences [44, 45] in the assessment of bone destruction in the pediatric population may cause either overstaging or understaging of JIA. For instance, bony depressions resembling erosions at the wrist may represent normal variation in children [46]. A group within the Health-e-Child (HeC) project and Outcome Measures in Rheumatology (OMERACT) MRI in JIA group published recommendations for the MRI protocol of the wrist in JIA patients, which led to facilitating wrist MRI evaluation and reducing misdiagnosis [47].
Attempts have been made to create a similar MRI summarized score for the ankle that enables discrimination between ankle arthritis in JIA from non-JIA patients with clinically suspected arthritis. The findings of Ostrowska et al. [48] confirm that MRI diagnosis of JIA remains a challenge, and with the exception of tendinitis, other MRI features are nonspecific for JIA, and thus the scoring system has proven inadequate.
Pharmacotherapy for JIA involves nonsteroidal anti-inflammatory drugs (NSAIDs), glucocorticosteroids (GCs), conventional and biologic disease-modifying drugs [49]. Currently, to assess the efficacy of treatment, a set of clinical and laboratory variables expressed by the American College of Rheumatology Pedi improvement index (ACR, Table IV.D.1-4) [50], the Juvenile Arthritis Disease Activity Score (JADAS) [51], and the ACR inactive disease criteria [52] are used. However, some clinical studies have demonstrated the effectiveness of MRI in accurately assessing the response to treatment of JIA. For example, a study by Malattia et al. [53] compared ACR pediatric response criteria with MRI features. Patients with JIA who achieved the highest level of clinical response had a significant reduction in synovitis on MRI, which was correlated with a lack of progression of structural damage.
Chronic non-bacterial osteomyelitis
Whole body MRI is one of the primary examinations used in the diagnosis as well as selection and monitoring of treatment for CNO [54]. Chronic non-bacterial osteomyelitis remains a very rare disease that mainly affects children and adolescents. Its prevalence is estimated at about 0.5–6 cases per 1,000,000 children with a female-to-male incidence ratio of 2 : 1 [55]. Chronic non-bacterial osteomyelitis manifests as osteoarticular pain, often at night, joint swelling and tenderness. In addition, generalized inflammatory symptoms such as fever, weakness and weight loss may be present [56]. Whole body MRI should be performed in any patient presenting with the above symptoms. This examination allows evaluation of both symptomatic and possible asymptomatic changes confirming the multifocal nature of the disease. A typical MRI change in the STIR sequence is a hyperintense focal inflammatory lesion adjacent to the growth plates of the long bones of the lower extremities and additional inflammatory lesions in the spine, pelvis, clavicle and/or sternum [57]. In addition, WB-MRI shows the distribution of inflammatory lesions, demonstrating a typical pattern of symmetrical and bilateral involvement, and provides information on disease activity and possible complications [58]. Moreover, WB-MRI can help detect asymptomatic vertebral compression, identify the optimal biopsy location or completely avoid an invasive procedure. It should be noted that patients with CNO often undergo invasive examinations such as bone biopsy to exclude neoplasms such as histiocytosis, Ewing’s sarcoma, osteosarcoma, leukemia or lymphoma before a definitive diagnosis is made [59]. With the introduction of WB-MRI, bone biopsy can be avoided in many patients in good general condition, with low elevation of acute phase proteins, involvement of multiple bones, typical radiologic findings and a favorable response to treatment with nonsteroidal anti-inflammatory drugs (NSAIDs).
First-line treatment in CNO includes NSAIDs, while corticosteroids, methotrexate, bisphosphonates, TNF-α inhibitors and IL-1 blockers can be considered second-line options, the choice of which depends on the location of the bone lesions, the presence of systemic symptoms and the patient’s clinical condition. Whole body MRI is also important for treatment selection or modification, and is recommended to assess the efficacy of CNO treatment at 6 and 12 months after initiation of pharmacotherapy [60].
Juvenile idiopathic inflammatory myopathies
Juvenile idiopathic inflammatory myopathies mainly include juvenile dermatomyositis (JDM) and juvenile polymyositis. In developmental age, the estimated incidence is 0.2–0.7/100,000/year, with a female predominance (3 : 1). The typical symptom is weakness of the proximal muscles, mainly of the shoulder and pelvic girdle, back and neck muscles [3, 61]. Magnetic resonance imaging of the musculoskeletal system is considered the best imaging diagnostic method for JIIMs, as it allows accurate assessment of soft tissue abnormalities, including bone [62, 63]. In 2013, a modification of the classic criteria for the diagnosis of JDM was proposed, in which MRI was established as the recommended diagnostic method. Currently, to diagnose JDM, typical skin lesions must be present and at least three of five criteria must be met, which include MRI lesions corresponding to myositis [64]. Typical changes present on STIR and T2FS sequences include regions of edema with increased signal intensity, which correlate with myositis of the fascia and subcutaneous tissue [65]. An example of WB-MRI in a patient with suspected myopathy is presented in Figure 2. With WB-MRI, it is possible to obtain information about the extent of the inflammatory process, as well as to select an appropriate biopsy site for the collection of material for histopathological examination, which is necessary to make a confident diagnosis of JIIMs [66]. In addition, monitoring of disease activity, response to pharmacotherapy and evaluation of possible adverse events, for example in the form of sterile necrosis or fracture, is achievable [67]. Adequate treatment mainly through the administration of very high doses of corticosteroids and immunosuppressive drugs and, in the absence of satisfactory improvement, intravenous immunoglobulins is aimed at preventing muscle atrophy. Fatty muscle degeneration seen in T1-weighted MRI sequences indicates muscle damage and may accompany the chronic form of JDM [68].
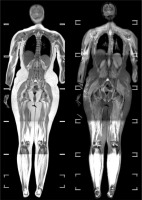
Fig. 2
Whole body MRI of a patient with suspected myopathy. On the left T2 sequence, and on the right STIR sequence. The muscles show features of diffuse signal enhancement in the STIR sequence images, suggesting the presence of an inflammatory process within them. Features of subcutaneous edema are noticeable in both arms.
Juvenile systemic scleroderma
Juvenile systemic scleroderma (JSSc) is a connective tissue disease characterized by chronic progressive fibrosis of tissues and organs. The prevalence of JSSc is approximately 3–6/million children. The clinical presentation is highly variable and can result from involvement of many systems, including musculoskeletal, respiratory, gastrointestinal, cardiovascular and very often skin. The most characteristic symptom of JSSc is Raynaud’s syndrome, which occurs in up to 90% of children [69]. The diagnosis of JSSc is based on the Provisional Classification Criteria (PRES/ACR/EULAR) of Juvenile Systemic Scleroderma [70]. Magnetic resonance imaging is one of the important diagnostic tools, as it can reveal abnormalities in the skin, subcutaneous tissues and deep tissues that are part of the criteria above. Magnetic resonance imaging of both the whole body and specific anatomical regions is performed, depending on the severity of the disease and symptoms. The most common MRI findings are inflammatory infiltrates and atrophy of subcutaneous tissue. In linear scleroderma “en coup de sabre,” in addition to the band-like sclerotic lesions typically involving the coronal and parietal regions of the scalp, children often have neurological symptoms, and therefore brain MRI is often performed. Myositis, fascial involvement and bone marrow edema are less common; however, MRI, particularly PDFS and STIR sequences, are also used for their identification. In addition, MRI can prove valuable in diagnosing cardiac involvement in JSSc. Furthermore, MRI appears to be particularly necessary in overlapping syndromes, which most often involve scleroderma and inflammatory myopathies [71]. Unfortunately, there is no causative treatment for JSSc, so individually targeted organ-specific treatment is used, the efficacy of which, depending on the systems involved, can be monitored by MRI.
Juvenile systemic lupus
Juvenile systemic lupus erythematosus (JSLE) is an autoimmune disease in which complex disorders of the immune system lead to a chronic inflammatory process in multiple tissues and organs [72, 73]. In children, the prevalence rate ranges from 0.36 to 0.9/100,000 children per year. Symptoms of involvement of multiple systems, including the kidneys, nervous system and hematopoietic system at the onset of the disease, are characteristic for SLE in children. Magnetic resonance imaging is useful in evaluating soft tissue inflammation, bone marrow edema and erosive bone lesions, and is the imaging modality of choice in the early diagnosis of certain complications such as bone necrosis, pathological fractures or osteomyelitis. Typical MRI changes in JSLE include the presence of edematous tendinitis and edema of the joint capsule [74]. In addition, brain MRI remains a significant feature in JSLE, and is performed in the case of coexisting neurological symptoms even though there are no specific neuro-radiological findings that are definitive for neuropsychiatric JSLE, and imaging can be normal, even in cases with small vessel central nervous system vasculitis [75]. Magnetic resonance imaging in children indicates that white matter hyperintensity is the most commonly observed lesion in patients with abnormal MRI findings [76]. It is noteworthy that white matter lesions have also been demonstrated in some patients without neuropsychiatric SLE, suggesting that these lesions are common, and their specificity remains to be determined [77]. Magnetic resonance imaging also allows quantitative and volumetric analysis of cerebral atrophy [76]. According to the SHARE Group’s 2017 guidelines, all patients with JSLE receive treatment based on hydroxychloroquine, GCs and disease-modifying drugs [78, 79]. Since juvenile systemic lupus erythematosus is a disease involving multiple organs, MRI should be widely used, not only for early diagnosis, but also to assess progression.
Artificial intelligence in magnetic resonance imaging
Artificial intelligence is a very promising tool that may also have wide application in rheumatology. Currently, more and more algorithms for evaluating MRI findings are being developed worldwide, offering potential applications in the diagnosis of rheumatologic diseases. To date, these methods have not yet found widespread use in clinical practice, but work is underway to refine and validate them for evaluating specific structures. In particular, algorithms to assess bone marrow edema in the sacroiliac joints or myositis have been created [80–83]. Artificial intelligence will certainly facilitate the work of radiologists in the future and potentially reduce the time required to describe a given examination. However, in order for it to be implemented in everyday use, it must first be tested on extensive, high-quality data sets.
Magnetic resonance imaging of either specific anatomical regions or the whole body is an important examination in the diagnosis of rheumatic diseases of developmental age.
The main disadvantages of this examination are its relatively high cost, limited availability, and duration of approximately 30–60 minutes [84]. This results in the need for sedation or general anesthesia in uncooperative children, especially those between 6 month and 6 years of age [85]. The use of anesthetics in children is associated with a potential risk of cardiorespiratory complications and side effects [86]. Therefore, various strategies have been developed to reduce sedation in pediatric patients. These strategies include: examination preparation with MRI simulation; asleep but not sedated techniques; awake and relaxed techniques using certified child life specialists, animal-assisted therapy, a child-friendly environment and in-scan entertainment; and non-sedated MRI protocol modifications such as shorter scan time, prioritizing sequences, reducing motion artifact, noise reduction, limiting use of Gd, employing an open MRI and modifying protocols [86, 87]. The introduction of patient-specific examination protocols to obtain diagnostically essential images minimizes the length of time the pediatric patient remains in the scanner [86] while application of open MRI may reduce the proportion of patients undergoing sedation due to claustrophobia [87].
Table I summarizes the described diseases and the imaging methods recommended in first line diagnostics.
Table I
Summary of the application of MRI of the musculoskeletal system in the diagnosis of rheumatic diseases in the pediatric population.
| Disease | Recommended MRI | Region of interest | Typical MRI findings |
|---|---|---|---|
| JIA | |||
| CNO | |||
| JIIMs | |||
| JSSc | |||
| JSLE |
[i] BME – bone marrow edema, CNO – chronic non-bacterial osteomyelitis, JIA – juvenile idiopathic arthritis, JIIMs – juvenile idiopathic inflammatory myopathies, JSLE – juvenile systemic lupus erythematosus, JSSc – juvenile systemic scleroderma, MRI – magnetic resonance imaging, WB-MRI – whole body magnetic resonance imaging.
Magnetic resonance imaging allows a more accurate assessment of the extent of the inflammatory process and disease activity than clinical examination alone. Magnetic resonance imaging of the musculoskeletal system is primarily a very accurate examination, which allows visualization of any anatomical region in multiple planes.
Conclusions
Magnetic resonance imaging allows the most accurate assessment of joints including ligaments, tendons, cartilage, nerves, blood vessels and bones, but also muscles. Although it has known limitations (costs, duration of examination, and in certain age-dependent cases the need for anesthetic intervention) it is a safe and precise imaging method that does not expose the patient to any ionizing radiation, which is particularly important in the pediatric population. It is the method of choice in the diagnosis of certain diseases, such as chronic recurrent multifocal osteomyelitis.




