Introduction
Hyperuricemia is an excess of uric acid in the body defined as an increase above the reference levels, i.e. 6 mg/dl in women and 6.8 mg/dl in men. High levels of uric acid can lead to deposition of sodium urate crystals in the joints, which leads to gout, leading to inflammation and, as a consequence, pain and limited mobility. An increase in uric acid (UA) in the blood causes an increase in its filtration by the kidneys, which predisposes to deposition of urate deposits, which result in urolithiasis. In addition, hyperuricemia is a recognized risk factor for the onset and progression of cardiovascular disease [1]. On the other hand, treatment of hyperuricemia can be associated with hypersensitivity reactions, which should be kept in mind when instituting therapy. It is important to assess the advantages and disadvantages before starting treatment. The aim of this review is to present elements of the management of hyperuricemia in daily clinical practice and to assess the risks and benefits associated with treating this condition.
Complication of increased level of uric acid
Acute kidney injury (AKI) can result from various sources of damage, such as restricted blood flow (ischemia), toxic substances affecting the kidneys (nephrotoxicity) or crystal formation. Regardless of the cause, AKI usually involves a common sequence of damage. It involves cell death in the renal tubules and infiltration of immune cells, leading to inflammation in the surrounding tissue and eventual scarring (fibrosis) [2]. Studies have shown that UA is involved in those processes in crystal-dependent and crystal-independent mechanisms [3]. Typical AKI caused by hyperuricemia is acute urate nephropathy. Most often it is seen as a complication of tumor lysis syndrome, when the UA level in serum rises above 12 mg/dl [4]. Without underestimating the seriousness of tumor lysis syndrome, this complication occurs quite rarely, which is why other reports from meta-analyses have attracted more attention. Individuals with elevated levels of UA demonstrated increased rates of AKI following percutaneous coronary intervention (PCI) and exposure to contrast agents compared to those with normal uric acid levels [5]. Another meta-analysis revealed that allopurinol preventively decreases the incidence of contrast-induced AKI among patients undergoing coronary angiography [6].
Chronic kidney disease (CKD) is an ongoing condition marked by both structural and functional alterations in the kidneys, stemming from diverse factors. In current international guidelines it is commonly identified by a deterioration in kidney function, indicated by an estimated glomerular filtration rate (eGFR) below 60 ml/min per 1.73 m2, or by evidence of kidney damage, such as albuminuria, hematuria, or abnormalities detected through diagnostic tests or imaging, persisting for at least three months [7]. Referring to hyperuricemia, the most commonly cited mechanism associated with UA-related eGFR decline is the increased presence of macrophages and pro-inflammatory factors [8, 9]. Other meta-analyses have shown a positive correlation between rising plasma UA and the number of new diagnoses of CKD, as well as with accelerated progression of the CKD [10–12].
Hypertension is inextricably linked to suboptimal kidney function and secretory function. Many studies have shown that hyperuricemia is an independent risk factor for hypertension [1, 13]. So far, several mechanisms for the effect of hyperuricemia on the development of hypertension have been described. It has been shown that mechanisms potentially contributing to hypertension such as endothelial cell activation of the renin-angiotensin system (RAS), inhibiting nitric oxide and inducing oxidative stress are dependent on urates [14, 15]. In extensive meta-analyses, an association between increased UA levels as an independent factor in the development of hypertension has been proven. It has also been proven that females and younger people with hyperuricemia are more likely to develop hypertension [16].
Type 2 diabetes mellitus (T2DM) is one of the most prevalent metabolic disorders globally, primarily arising from two key factors: impaired insulin secretion by pancreatic β-cells and reduced responsiveness of insulin-sensitive tissues to insulin [17]. The link to the development of T2DM was suggested more than 70 years ago [18] and has been confirmed many times since then [15, 19]. In one clinical study, long-term, asymptomatic hyperuricemia in young people was shown to be an independent factor in the development of both insulin resistance and T2DM [20]. Subsequent analyses confirmed these results – it was estimated that the overall relative risk could range from 6 to 11% for every 1 mg/dl above the upper limit of normal for UA in the blood [21, 22]. It should also be remembered that hyperuricemia is a factor which accelerates the progression of microvascular and macrovascular complications of diabetes [23, 24].
Treatment of hyperuricemia
Before embarking on treatment, it should be determined whether it is necessary. An absolute indication for the implementation of pharmacotherapy is the persistence of UA above 12 mg/dl [25]. Treatment independent of uric acid levels is also mandatory when gout or urolithiasis is diagnosed. However, the situation is less obvious in patients with hypertension and high cardiovascular risk.
As discussed before, the association of hypertension and hyperuricemia has been widely documented [26, 27]. The incidence of hyperuricemia comorbidity in hypertension is several times higher than in the normotensive population. Impaired renal excretion of UA is thought to be the cause of this condition [27]. In addition, another cause of hyperuricemia among patients with NT is the treatment of hypertension itself. Hypotensive drugs, especially thiazide-like diuretics, often increase blood UA levels [28]. It is also important to consider the potential benefits of antihypertensive drugs that reduce uric acid levels. Losartan, an angiotensin II receptor blocker, has been shown to have a hypouricemic effect [29].
If asymptomatic hyperuricemia is diagnosed, attention should be paid to cardiovascular risk. An available tool to determine cardiovascular risk is the SCORE 2 scale (European Society of Cardiology 2021 guidelines), which is easy to apply and helpful in the physician’s everyday practice [30]. The scale makes it easy to assign a patient to the appropriate risk group. Factors that are taken into account are: age, gender, nicotinism, systolic blood pressure, and non-HDL cholesterol level (defined as the difference between total cholesterol and HDL levels). With a score defined as high or very high risk (greater than 2.5%/7.5% for those < 50 years of age and greater than 5%/10% for those > 50 years of age on the SCORE2 scale, respectively), the initiation of hypouricemic therapy should be considered [31, 32].
The first step in therapy is to educate the patient about eliminating from the diet products with a high content of purines, from which UA is formed in the body. Meat, offal, seafood, and alcohol, especially beer and strong liquors, should be avoided [33]. Then, after ruling out contraindications to pharmacotherapy, the drug and dose should be selected. The standard regimen initially uses allopurinol at a dose of 100 mg/day and gradually increases the dose to 300 mg/day depending on the persistence of UA levels. However, the recommendations are different in hypertensive patients, in whom damage to renal function can be expected. Inclusion of standard treatment in such patients may be associated with hypersensitivity reactions to the drug. In such cases, a lower dose of 50 mg/day can be used and then modified until satisfactory results are obtained [32, 34].
An alternative to the use of allopurinol is febuxostat. It is an effective therapeutic option for managing hyperuricemia as a xanthine oxidase inhibitor. The initial recommended dose is typically 40 mg once daily, with dose escalation to 80 mg considered, if necessary, after 2–4 weeks and increased up to 120 mg. Close monitoring of renal function, blood pressure, and uric acid levels is paramount in patients with hypertension and CKD [35]. This medication carries a risk of exacerbating hypertension and associated adverse effects. In dose escalation for these patients, it is important to exercise caution. Regular clinical assessment is imperative to monitor response and potential therapy modifications. Optimizing febuxostat dosing in such cases requires a delicate balance between therapeutic efficacy and mitigating adverse events. According to studies, it is less toxic and allows for better results. It is also recommended treatment of hyperuricemia by the American Journal of Hypertension [36].
Hypersensitivity to allopurinol
Hypersensitivity reactions to allopurinol are not really common among healthy individuals. Most of them are a mild form called maculopapular eruption (MPE), also known as a drug induced rash [37]. According to research, no more than 2% of patients treated with allopurinol develop this type of reaction [38]. More severe reactions called severe cutaneous adverse reactions (SCAR) includes Stevens-Johnson syndrome (SJS), toxic epidermal necrosis (TEN), drug reaction with eosinophilia and systemic symptoms (DRESS) and allopurinol hypersensitivity reaction (AHS).
Stevens-Jonson syndrome and toxic epidermal necrolysis
A potentially life-threating condition after allopurinol use can be an acute skin reaction known as SJS [39]. It manifests as a blistering rash, with erosive lesions on the mucous membranes of the nose and throat. Typically less than 10% of the body surface is affected [40]. Flu-like symptoms may also occur. When more than 30% of the body surface is involved, it is called toxic epidermal necrolysis (TEN) [41]. According to academic research, allopurinol use is the most common cause of this syndromes in Europe [42] and 6 per 100,000 new allopurinol users develop this syndrome [43]. Depending on the severity of SJS/TEN the mortality rate is between 7.5% and 23% [44, 45] and it is lower when the etiological agent is known and when treatment has been administered quickly [46]. The SJS/TEN treatment consists of systemic administration of corticosteroids, thalidomide and, in severe cases, immunoglobulins. Patients should be cared for in highly specialized centers [47].
Drug reaction with eosinophilia and systemic symptoms
Another severe allergic reaction that can occur in patients using allopurinol is DRESS [48]. It is characterized by fever, skin lesions in the form of maculopapular-pigmented skin rashes, lymph node enlargement, involvement of internal organs and changes in the peripheral blood (eosinophilia, neutrophilia, neutropenia, thrombocytopenia, hemolytic anemia). It is a rare complication, but like SJS/TEN with a high mortality rate of about 10% [49].
Allopurinol hypersensitivity syndrome
One of the most dangerous reactions to be aware of during allopurinol therapy is AHS. It usually reveals itself in the first 3 weeks after starting therapy [50]. It manifests with rash (erythema multiforme, diffuse maculopapular rush) and is accompanied by additional symptoms such as liver damage, kidney damage, and fever) [43]. The syndrome is reported to occur in about 1 in 1,000 to 1 in 10,000 patients and mortality is estimated between 20 to 25% [51, 52].
HLA-B*5801 – genetic factor
Studies have revealed that hypersensitivity to allopurinol is not equally reported worldwide. This suggests that there is a genetic predisposition to sensitivity. In 2005, an association was discovered between the risk of SCAR after allopurinol therapy and HLA-B*5801 [53, 54]. Subsequent studies have demonstrated that possessing a single allele of this gene raises susceptibility to hypersensitivity reactions in comparison to a population without this gene, whereas possessing two alleles further increases the risk of hypersensitivity reactions [55]. The gene is most prevalent in East Asia (approximately 20%), less so in Europe (approximately 5%), and least prevalent in Japan (approximately 0.6%) [53, 56, 57].
Case description
As an example of problematic treatment with allopurinol is a case of a 74-year-old woman with multimorbidity (chronic hypertension, allergic bronchial asthma, gastrointestinal reflux, chronic gastritis, neuromuscular bladder dysfunction, degenerative changes of the spine, osteoporosis and chronic hepatitis carrier).
This patient presented to the general practitioner with a skin rash suspected to be caused by sulfamethoxazole with trimethoprim, which had been prescribed by a physician 12 days prior the visit due to diagnosis of urinary tract infection. The patient discontinued the medication 4 days before the consultation. Also due to elevated blood uric acid levels (6.1 mg/dl) and co-morbid hypertension, 100 mg per day of allopurinol had been started almost a month earlier.
Apart from the rash, the patient was in good overall health and exhibited no symptoms related to the circulatory or respiratory systems. The patient presented with pustular lesions that were observed throughout her body and blanched under pressure. No other cutaneous changes were noted.
In the recommendations from the doctor, the patient received fexofenadine 180 mg 1 × 1 and dimethindene topically, hydration at least 2 l/day, rest, observation of the health condition, and in case of lack of improvement and recurrence of symptoms, contact with a doctor.
After 3 days, the patient went to the family doctor again due to persistent rash and severe itching of the trunk and all limbs. In addition, the patient reported a problem with shortness of breath. On the second examination, the measles changed to maculopapular rash with the presence of blisters with no separation of epidermis. Examination of the throat revealed mucosal congestion. In measurement of vital signs, temperature 37.2°C, tachypnea 25/min, tachycardia 110/min, and blood pressure 140/90 mmHg were observed. Assuming a drug-induced allergic reaction, treatment with dexamethasone 4 mg intramuscularly (i.m.) and a first-generation H1 histamine antagonist (antihistamine) with anticholinergic properties, clemastine 2 mg i.m., were administered. The doctor decided to discontinue allopurinol, due to the frequent occurrence of skin rashes following the use of this drug. After receiving treatment and discontinuation of allopurinol, the skin changes and other symptoms in 24 hours totally resolved. The laboratory tests at this moment were not available to the clinician.
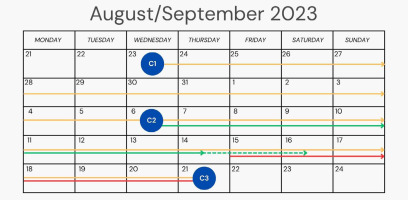
Analyzing the patient’s history, it is worth noting that the allergic rash after allopurinol did not occur immediately. From taking the first dose of the drug on the 23rd of August (Figure 1 – consultation 1 – C1, yellow line on the chart) to the first symptoms of allergy to the drug, it took more than 3 weeks – 15.09 (Figure 1 – red line). The applied treatment with sulfamethoxazole with trimethoprim (Figure 1 – 6.09, C2) should have been continued for 10 days (Figure 1 – until 16.09, green dashed line), but was discontinued on 14th of September.
Discussion
The risk factors for developing allopurinol-induced hypersensitivity reactions including TEN and SJS are as follows: the recent introduction of this drug with a higher starting dose, older age, presence of the HLA-B*58:01 allele, use of diuretics, and renal impairment. In the case of the present patient, some of the above-listed risk factors were observed and in addition the allergic disease as bronchial asthma may have increased the risk of reaction to allopurinol [58]. No significant drug interactions were found when sulfonamide was used concurrently with allopurinol; however, both of these drugs carry the risk of severe skin reactions, both TEN and SJS, which may be life-threatening clinical situations, especially TEN. The severity of these skin reactions depends on age (> 40), heart rate (> 120 beats/minute), and separation of epidermis on more than 10% of the body surface area (BSA) on the first day of such symptoms. Other risk factors of poor prognosis are previous history of cancer, diabetes and laboratory results such as blood urea nitrogen > 28 mg/dl, glucose > 252 mg/dl (14 mmol/l), and bicarbonate < 20 mEq/l [58–60].
The risk of side effects following allopurinol administration could be high, particularly when commencing treatment with a dosage exceeding 100 mg/dl, or when patients present with concomitant chronic conditions such as hypertension and/or chronic kidney disease. However, the allopurinol drug-induced reactions, especially SJS and TEN, are more common in Asian patients with genetic predisposition (HLA-B*58:01) [61]. In the present scenario, it is imperative to acknowledge the patient’s multimorbidity and concurrent use of multiple medications from diverse therapeutic classes. In such complex cases, it is prudent to consider initiating allopurinol therapy at a lower dose, such as 50 mg, to mitigate potential adverse reactions.
Conclusions
In daily rheumatological practice, hyperuricemia is usually connected with gout. However, the holistic approach for all patients treated for hyperuricemia for other reasons and by other specialists should always be considered. All clinicians should carefully analyze the patient’s condition and consider whether initiating uric acid-lowering therapy will be more beneficial to the patient than the potential side effects of treatment. Some risk factors for allopurinol-induced reactions can be assessed without additional tests, e.g. age, knowledge of comorbidities or taking other medications. Therefore, regardless of the rheumatological diagnosis, patients considered for uric acid-lowering therapy require careful management and close monitoring for potential drug-related adverse effects.