Introduction
Polymyalgia rheumatica (PMR) is estimated to be the most common inflammatory rheumatic disease in older adults. Age 50-plus is a diagnostic and classification criterion. Worldwide, PMR incidence increases until the age of 90 with a peak around the age of 75 [1–5]. The onset of PMR in a centenarian man has been reported [6].
At present, no specific laboratory tests are available; therefore, the diagnosis of PMR is essentially clinical. Typically, PMR patients complain of sudden-onset pain in the shoulder and pelvic girdles, sometimes accompanied by neck ache, and of morning stiffness lasting more than 45 minutes. All self-care activities of daily living (ADL) involving the shoulder and pelvic girdles are restricted to the point of causing significant disability.
Additional manifestations such as fever, general discomfort, fatigue, loss of appetite, and loss of weight can be present in some patients [7, 8]. Inflammatory markers such as erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP) concentrations are usually raised at the time of diagnosis, but normal ESR and CRP should not be reasons of exclusion for diagnosis of PMR [9–11]. Glucocorticoids (GCs) are the cornerstone of PMR treatment [12].
In 1979, Bird et al. [13] first proposed a 7-item diagnostic criteria set:
age > 65 years,
ESR more than 40 mm/hour,
bilateral shoulder pain and/or stiffness,
stiffness > 1 hour,
duration onset > 2 weeks,
depression and/or weight loss,
bilateral upper arm tenderness.
The authors indicated that a diagnosis of PMR was probable if any three or more of these criteria were fulfilled. Depression was considered a diagnostic criterion but seemed to be measured by “rule of thumb”, showing a sensitivity of only 29% in the validation study.
Some authors listed age of 65-plus as a diagnostic criterion, whereas subsequent proposal guidance widened the age criterion to over 50 years [14-17]. Depression has never been assessed by any diagnostic or classification criteria for PMR published after 1979.
On the other hand, depression had been reported commonly in papers referring patients with PMR, before 1979 [18].
More recently, the significance of depression in PMR patients has been re-underlined, and some researchers have suggested that PMR patients may be at an increased risk of depression. The long-standing course of PMR, its inflammatory nature, and the GC treatment may be major contributing factors [19, 20].
Additionally, depression may per se influence patients’ therapeutic adherence. In selected subjects, depression can be worsened by GCs [21, 22], and this can be a serious obstacle for the continuation of GC therapy. Especially in these subjects, the assessment of depression as a patient-related outcome (PRO) would be very important.
The aim of our article is to evaluate the relationship between PMR and depression, the impact of depression in PMR patients as a PRO, and its overall effect on healthcare provision.
Material and methods
Systematic literature searches were performed on 19th and 20th May 2020 based on preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [23]. The three main bibliographic databases: EMBASE, MEDLINE (OVID interface) and PsycINFO (via the NICE HDAS interface) were explored by an author (Isetta).
In order to maximize sensitivity, the search strategies relied on blended subject headings and key word (free text) approaches, with the search language adapted to each database’s specific syntax. On MEDLINE, MESH headings were supplemented by natural language: (POLYMYALGIA RHEUMATICA/OR “polymyalgia rheumatic*.af) AND (exp. DEPRESSIVE DISORDER/OR depress*af OR FATIGUE/OR fatigue*af OR MUSCLE WEAKNESS/OR weakness/OR exp. SLEEP WAKE DISORDERS/OR PATIENT REPORTED OUTCOME MEASURES/OR PROMS.ti,ab OR GLUCOCORTICOIDS/ae).
Inclusion criteria
The search was restricted to all studies and case-reports with an English abstract, published in any language, since 1979 (when depression was first proposed as diagnostic criterion for PMR by Bird et al.) describing the association of PMR with depression.
Exclusion criteria
Reviews, conference abstracts, comments, and non-original articles were excluded, but each review’s reference list was scanned for additional publications meeting this study’s inclusion criteria. When papers reported data partially presented in previous articles, we referred to the most recent published data.
Articles discussing giant cell arteritis (GCA) and PMR, when data and observations for the two conditions were not clearly subdivided. Giant cell arteritis is closely linked to PMR: 40–60% of GCA patients show signs of PMR, whereas 10–16% of PMR patients can have GCA findings. Nevertheless, PMR and GCA are two different diseases and their association has major therapeutic and prognostic consequences.
In accordance with the PRISMA 2009 checklist, the full search strategy for one database (MEDLINE) is detailed in the supplemental materials and methods section.
Data extraction
Three of the authors (Serra-Mestres, Nizama-Via, and Manzo) independently reviewed the titles and abstracts of all identified citations. After reviewing the abstracts, data comparisons were conducted to ensure completeness and reliability.
The inclusion criteria, data on study design, source of information, and participant characteristics were independently extracted. Full-text versions of potentially relevant papers identified in the initial screening were sourced. Differing decisions were resolved by consensus.
Quality and bias risk assessment
A subjective assessment of the methodological quality of observational studies was performed by all the authors using the Newcastle-Ottawa scale, which is a quality assessment tool for non-randomized studies, endorsed for use in systematic reviews of non-randomized trials by the Cochrane Collaboration [24].
The Newcastle-Ottawa scale uses a “star system” based on three major criteria: study groups’ selection (0–4 stars, or 0–5 stars for cross-sectional studies), comparability of the groups according to key and additional factors (0–2 stars), and determination of the outcome of interest or exposure (0–3 stars).
A total score of 3 or less was considered poor, 4–6 was considered moderate, and 7–10 was considered high quality. Studies scoring 3 or less were excluded from our review.
Results
Description of included studies
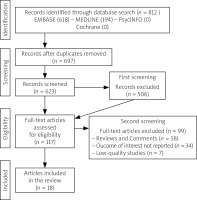
As reported in Figure 1, the initial search yielded 812 papers, of which 115 duplicates were excluded. A total of 697 articles had a first screening, and 506 were excluded based on title and abstract reviews; 117 articles underwent a full-length review, and 99 full-text articles were excluded because they did not meet the inclusion and exclusion criteria (reviews and comments = 58; articles with outcome of interest not reported = 34; low-quality articles = 7). Finally, 18 articles were included in the review. Their study design and outcomes concerning depression and/or depressive symptoms are reported in Table I.
Table I
Study design and outcomes concerning depression and/or depressive symptoms of the 18 selected articles in the review
None of the studies reviewed provided evidence for a diagnosis of depression having been reached by careful history-taking and application of accepted diagnostic criteria; hence, most studies were most likely discussing depressive symptoms either reported via rating scales or directly by patients. This clearly questions the reliability of any assertions about patients suffering from depression.
Depression as comorbidity
The term “comorbidity” may refer to two or more pathologies that coexist simultaneously but independently of each other, or it applies to pathologies that appear secondarily to the onset of an underlying pathology.
Our literature search indicates that every account of the relationship between PMR and depression refers to the second possibility. For instance, psychological comorbidities (and depression among these) are common following the diagnosis of a chronic illness like PMR. The possibility that depression can be induced by GCs is discussed in a separate paragraph. From this literature review, the following findings are now presented.
The reported prevalence of depressive symptomatology in PMR varies from 2 to 29% [25]. The small sample size of published studies and the fact that they were conducted in secondary or tertiary care settings should be considered as a potential bias [19, 26, 27].
Furthermore, the elderly, commonly affected by PMR, often have multiple comorbidities. In a 2018 cross- sectional United Kingdom study in primary care settings, the authors reported a higher prevalence of current depressive symptoms among primary care PMR patients in comparison with the general older adult population. In this study, risk factors for depression included female sex, younger age, and presence of glucocorticoid- related comorbidities. In a multivariable analysis, only diabetes remained significantly associated with depression [19].
Instead, a systematic review published in the same year found no evidence of links between PMR and psychiatric comorbidities, depression included. The authors highlighted how research into comorbidities in PMR was, overall, methodologically weak. For instance, many of the cohort studies identified failed to include comparison groups; almost half of them sourced their PMR patient sample from hospital discharge data, and most studies restricted their focus to a small number of comorbid conditions [28].
To the best of our knowledge, no observational study of the prevalence of depression meeting standardized diagnostic criteria before the diagnosis of PMR has been published.
Depression as a polymyalgia rheumatica-mimicking disease
Several PMR-mimicking illnesses have been reported. Their exclusion is mandatory, as underlined by the latest guidelines by the American College of Rheumatology and the European League Against Rheumatism (ACR/EULAR) collaborative initiative [18]. It should not be ruled out in clinical practice that patients initially diagnosed with PMR may be reclassified as having a different disease during follow-up [29–31].
According to this review, depression was never considered as a PMR-mimicking disease, but fibromyalgia-related depressive manifestations can be an accompanying feature in many patients affected with PMR [32–34].
Depression as patient-reported outcome
Patient-reported outcomes (PROs) provide a means for patients to communicate effectively with their care teams about their disease. In addition, PROs have been proven to add valuable and unique information on treatment efficacy and quality of life that is immediately relevant to the management of their disease activity [35, 36].
In 2017, an Outcome Measures in Rheumatology (OMERACT) special interest group published data regarding patient-reported pain and stiffness. Health Assessment Questionnaires (HAQ) were used, but they do not directly address depression [37].
In other previous studies on quality of life in PMR, short form (SF) questionnaires were used and although these questionnaires contain depression-related domains, they do not directly address depression [38, 39].
Patient-reported outcomes are frequently applied as a screening tool to diagnose depression, even though results from self-scored questionnaires’ risk overlapping with symptoms of somatic diseases, thus limiting their usefulness. For instance, Vivekanantham et al. [19] reported that increased PROs for depression were found only in PMR patients with comorbidities.
Patient-reported outcomes were not mentioned in EULAR/ACR recommendations for the management of PMR [12], and a recent OMERACT study did not include depression among PROs [40].
Finally, depression was not included in traditional PMR activity scores. For instance, the Visual Analogue Scale (VAS) of pain by patients remains the only PRO in PMR activity score proposed by Leeb and Bird [41].
Depression and inflammation
The possibility that inflammation plays a significant role in the genesis and maintenance of depression has frequently been reported and discussed in the literature.
This systematic review did not find any studies that specifically investigated the relationship between depressive disorder and inflammatory markers that could suggest (or not) a possible inflammatory etiology of depression in PMR.
The following two studies provide some peripheral evidence, even though their ascertainment of depressive features is not robust.
In a study of 102 PMR patients, levels of the anti-inflammatory cytokine interleukin 10 (IL-10) were measured. Compared to those with normal levels of IL-10, patients with high serum levels of IL-10 (> 7.79 pg/ml) presented with significantly fewer symptoms of PMR (and symptom score) such as adynamia, muscle pain in shoulders, upper arms, neck, and pelvic girdle, headache, morning stiffness, initial weight loss, and also depression.
The reported rates of symptoms of depression were 40.5% in the normal IL-10 level group and 18.3% in the high IL-10 group. There was no difference in GC treatment in the high IL-10 group compared to the normal IL-10 one in terms of daily dose and length of treatment. This study did not assess for depression in a standardized way and used a 33-item questionnaire that, amongst other issues, addressed current symptoms. A disease symptom score which included depression was then obtained [42].
Another study of 102 PMR patients (32 with recent-onset and 70 with chronic disease), investigated the role of prolactin (PRL) in relation to the number of typical symptoms of PMR and serum markers of systemic inflammation, namely IL-1 receptor antagonist, interleukin 2 (IL-2) and interleukin 6 (IL-6), tumour necrosis factor (TNF), soluble IL-2 receptor (sIL-2R), and soluble vascular cell adhesion molecule (sVCAM).
This study did not assess for depression in a standardized way and used a 33-item questionnaire that, amongst other issues, addressed current symptoms. A disease symptom score, which included depression, was then obtained. Symptoms of depression were reported in 27.5% of patients.
The study found that the number of PMR symptoms reported correlated positively with PRL levels, but that this was not the case in relation to serum markers of inflammation (those listed above in addition to CRP and ESR), suggesting that high levels of PRL do not seem to have a proinflammatory role in patients with PMR.
Patients with symptoms of depression or a higher number of disease manifestations had elevated serum PRL compared to patients without symptoms of depression or fewer symptoms of PMR [43].
Depression as an adverse event in older persons treated with glucocorticoids
The association between mood disorders and levels of glucocorticoids (GCs) has long been recognized, and it is common knowledge that this association is strictly linked to GC dosage and to exposure time [21, 44, 45].
A meta-analysis assessing the adverse effects of GCs in three different inflammatory diseases (rheumatoid arthritis, PMR, and irritable bowel syndrome) showed that reported rates were comparable. However, psychological and behavioral adverse effects (steroid psychosis, minor mood disturbances) accounted for the highest rate (20% of all side effects associated with GCs, 6% in PMR) [46].
In the group of patients under GC treatment studied by Vivekanantham et al. [19], those suffering from diabetes mellitus were more likely to show depressive symptoms. This suggests that frequent adverse effects of GCs, like diabetes, could raise the risk of developing depressive symptoms in GC users.
Another study reported insomnia, anxiety, and weight gain as adverse effects in PMR patients receiving steroid- sparing combination therapy with prednisone and methotrexate; however, it found no statistical association [47]. Guidelines recommend avoiding evening doses of GCs for the treatment of PMR because they can cause disruption of circadian rhythm and sleep disturbances [12].
However, circadian rhythms had been reported in PMR patients, justifying the usefulness of delayed-release prednisone taken at bedtime (administrated approximately at 10 p.m.) [48]. Indeed, the balance between advantages and disadvantages must always be taken into account in clinical practice.
Finally, other studies found that some patients treated with GCs report a “steroid high” or a burst of energy that later “wore off”, suggesting that GCs could potentially have a direct impact on mood in the short term [21, 49].
Different GCs types and regimens might trigger depression to varying degree. A 2-period, cross-over randomized controlled trial (RCT) that compared 2 dose regimens (daily vs. alternate) for deflazacort against methylprednisolone in patients with PMR reported that one (6.25%) patient in the 6-week daily deflazacort group (n = 16) developed depression, although there was no clear explanation as to how depression was diagnosed. No cases of depression or depressive symptoms were reported in the alternate-day deflazacort group or in the methylprednisolone group. This could suggest that chances of developing depression might be increased by daily rather than alternate doses of glucocorticoid [50].
The relationship between dose reduction or discontinuation of systemic GCs and improvement in depressive symptoms has been reported in PMR patients. Hoes et al. [46] suggested that high doses are associated with the occurrence of neuropsychiatric side effects, but low and medium dosages (defined as 30 mg prednisolone equivalent per day) seem to show no difference [46].
However, depression is associated with long-term (> 3 months) GC therapy at lower doses. These reports confirmed conclusions already highlighted in previous reviews regarding non-PMR patients [51].
With regard to administration routes, a trial of depot methylprednisolone compared with oral prednisolone in the treatment of PMR reported that only 1 (3.3%) out of 60 patients on oral prednisolone reported depression. Conversely, no patient reported depression in the depot methylprednisolone group. Although the numbers were too small to perform a statistical analysis, it would seem that administration routes are not a factor in inducing depression [52].
Again, neither of these two studies explained how a diagnosis of depression was reached, and the authors mentioned only the depressive symptoms reported by the enrolled patients.
Discussion
Depression affected more than 241 million people in 2017 [53]. However, its prevalence and severity grades vary according to different diagnostic criteria, rating scales, or self-reports.
For instance, a 2017 population study [54] found that the prevalence of any depression was 4.2% (moderate/severe: 1.6%) for the International Statistical Classification of Diseases and Related Health Problems. Tenth revision (ICD-10) [55], 9.3% (major: 2,1%) for Diagnostic and Statistical Manual IV-revised edition (DSM-IV-R) [56], 10.6% for the Montgomery Asberg Depression Rating Scale (MADRS) [57], 9.2% for the Geriatric Depression Scale-15 item (GDS-15) [58], and 9.1% for self-report.
The described scales are useful for classification purposes but do not offer a key to an unambiguous diagnosis, only attainable through clinical assessment.
Indeed, clinical assessment is the agreed diagnostic gold standard. Consequently, studies based on scales alone lack diagnostic accuracy, thus impacting on the validity of the association between any diseases (PMR among these) with depression.
As shown by our literature review, this methodological approach is constant, and should be modified in subsequent ad hoc research studies.
Another significant discussion point emerging from all included studies is that a depressive state is hard to define and measure in PMR patients. For instance, some physical symptoms leading to impairment of ADLs may enhance final scores due to classical manifestations of active PMR.
A short analysis of the Beck’s Depression Inventory (BDI) may serve as an example [59]. Most of the Beck’s Depression Inventory questions (at least 11/21) address symptoms that overlap with manifestations of increased PMR activity: being discouraged about the future, getting satisfaction, crying, being irritated, losing interest in other people, effort used to get started at doing something, being too tired to do anything, losing appetite, losing weight, being worried about health, and losing interest in sex.
It is common knowledge that aging is an important predictor of co-morbidity. In PMR, quantification of the prevalence of co-morbidities could help to identify patients who may be more difficult to treat.
However, this review highlighted methodological weaknesses and the presence of potential inclusion or referral biases in research into comorbidities in PMR so far [28, 60].
Failure to include depression among PROs (another key find) must also be highlighted. In 2014, an OMERACT special interest group began working toward the development of a core domain set for PMR [61].
A core outcome set is a list of domains that should be measured in every randomized controlled trial. Adoption of a core outcome set is important because it increases consistency across clinical trials, reduces selective-reporting bias, and increases the likelihood that trials will measure relevant and important outcomes [36]. To date, depression is rarely and inadequately assessed [34, 39, 40].
As already reported, depression was not included in traditional PMR activity scores. The Visual Analogue Scale (VAS) of pain by the patient remains the only PRO in PMR activity score. This is another critical point, because, even if pain is the most frequent PRO, its correlation with depressive symptoms was infrequently reported in published literature [34, 38]. Therefore, whether depressive symptoms or depression are related to pain rather than – for example – GC therapy or other different conditions is still unclear.
Indeed, depressive manifestations may appear during GC treatment. The association between mood disorders and levels of GCs has long been recognized [44, 45]. It is known that the human brain has specific receptors for corticoids: mineralocorticoids and glucocorticoids. Glucocorticoid receptors are mainly expressed in the hippocampus and the amygdala, and they are occupied by physiological levels of glucocorticoids. The latter are more widely expressed in stress-regulating centers such as the hippocampal–amygdala circuitry and the ascending aminergic neuronal networks. Both of them are cellular nuclear receptors that create a balance that contributes to the homeostasis of the brain. Thus, glucocorticoids act directly on neurons, guiding their structural and functional changes, an expression of “neuronal plasticity” [21]. The timing of psychiatric intervention is another critical issue that needs addressing.
Finally, as already highlighted, depression has never been assessed by any diagnostic or classification criteria for PMR published after the 1979 Bird criteria. After more than 40 years, the Bird’s criteria have proven to have very good performance, but the diagnostic role of depression – as our literature search highlighted – is far from clear and defined.
Conclusions
From this systematic review, an overall lack of robustness and accuracy emerged in the diagnosis of depression in PMR patients because rating scales were used rather than targeted clinical interviews and/or standardized diagnostic criteria, causing confusion between depression and depressive symptoms.
Currently there is no clear indication of the prevalence of depression in PMR patients. Other grey areas deserve to be highlighted, namely:
to date, no observational study of prevalence of depression before diagnosis of PMR has been published,
depression is not adequately considered as PRO, despite depressive symptoms often being reported by PMR patients,
depression is not included in PMR activity scores, despite the fact that it may per se have an impact on patients’ therapeutic adherence,
the studies regarding depression as an adverse event during GC treatment are scarce and with small sample sizes,
collaboration of different professional disciplines was anecdotal and should be improved through shared guidelines and methodologies.
We hope that some of these issues will be addressed when designing new studies.
Comments
The supplementary materials are available in the online version of the published article, but it is not considered as an integral part of this article and cannot be formally cited. Supplementary material is available for free with an article on-line version, in the format supplied by the authors, and is accessible to all readers. The responsibility for scientific of supplementary materials depends on the authors.
This work was accepted as original research with analysis data confirmed in supplementary material. Considering the breadth of the publications included in the analysis, 61 items of the references were exceptionally accepted by the editors’ decision.