Introduction
Rheumatoid arthritis (RA) is an arthritis type where inflammation in the synovial tissue damages joints and causes disability. Recent advancements in early diagnosis, targeted treatment, and expanded therapeutic options of disease-modifying antirheumatic drugs have substantially enhanced the management and long-term prognosis of RA [1]. To date, there has been no comprehensive epidemiological study conducted on patients with RA in the Republic of North Macedonia. Data from the University Clinic for Rheumatology in Skopje reveal a prevalence of 240 cases per 100,000 individuals and an annual incidence rate of 16.1 per 100,000 adults. While the prevalence of RA in 2017 was 1,200 cases, in 2018 the total number of RA cases increased to 1,300.
The 2010 American College of Rheumatology/European Alliance of Associations for Rheumatology (formerly the European League Against Rheumatism; ACR/EULAR) diagnostic criteria include number and size of affected joints, serology including rheumatoid factor (RF) and/or anti-cyclic citrullinated protein antibodies (ACPA), symptom duration (whether < 6 weeks or > 6 weeks) and acute-phase reactants such as C-reactive protein (CRP) and/or erythrocyte sedimentation rate (ESR) [2]. The EULAR recommends initiating therapy with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) as soon as a diagnosis of RA is confirmed. If there is no adequate response to this therapy within 3 to 6 months, especially for patients with poor prognostic factors such as presence of autoantibodies, high disease activity, early joint damage, or failure with two csDMARDs, the addition of a biological DMARD (bDMARD) or Janus kinase (JAK) inhibitor to the csDMARD is recommended. If this approach proves ineffective, switching to another bDMARD (from the same or different class) or a targeted synthetic (ts) DMARD is advised [3]. For this subset of patients, therapeutic alternative treatment includes transitioning from one tumor necrosis factor (TNF) inhibitor to another or adopting newer biologic agents with different mechanisms of action. These may include TNF inhibitors such as etanercept, infliximab, adalimumab, certolizumab, or golimumab, T-cell-targeted therapy (cytotoxic T-lymphocyte associated antigen 4 antibody – CTLA-4 – abatacept), interleukin-6 (IL-6) receptor antagonists – tocilizumab, or drugs exhibiting anti-CD20 activity such as rituximab (RTX) [4, 5]. Rituximab, a chimeric monoclonal antibody, selectively binds to the CD20 antigen on B cells. Its Fab domain interacts with CD20, while the Fc domain can induce immune effector functions, leading to B cell depletion through complement-dependent cytotoxicity (CDC) via C1q binding and antibody-dependent cell death through Fcγ receptors on granulocytes, macrophages, and natural killer cells. Rituximab is approved for the treatment of adult patients with severe active RA who have not responded adequately to, or have been intolerant to, other DMARDs, including one or more TNF inhibitor therapies. When used with methotrexate (MTX), RTX has been shown to slow the progression of joint damage and improve physical function. A single course of RTX consists of two intravenous infusions of 1,000 mg or two intravenous infusions of 500 mg, with a recommended time interval between the two infusions of 2 weeks. The need for further therapy should be determined 24 weeks after the previous therapy. Re-treatment should be applied if residual disease activity remains; otherwise, re-treatment should be delayed until the disease is reactivated. Available data indicate that a clinical response is usually achieved 16–24 weeks after the first course of treatment [6].
Response to RA treatment varies widely among individuals, influenced by factors including serum drug concentration, pharmacokinetics, and patient characteristics such as age, sex, renal and liver function, body mass index (BMI), and smoking. Furthermore, the clinical response is contingent on the disease’s condition and characteristics, with distinct RA subtypes such as seropositive or seronegative RA [7–10]. Research indicates factors that can predict the response to RTX, leading to the development of personalized therapeutic approaches. Baseline Disease Activity Score with 28-joint count (DAS28) index, the serological status for biomarkers such as RF or ACPA and comorbidities have been identified as significant factors impacting the effectiveness of RTX therapy [11–17]. The variability in individuals’ responses to RTX therapy leads to challenges in determining the optimal timing for retreatment. Prolonged intervals between treatment cycles increase the risk of relapses, diminished quality of life, and potential irreversible joint damage. On the other hand, shorter treatment intervals may increase the risk of side effects due to continuous B-cell depletion [18]. To address this, the DAS28 index serves as a tool for standardized retreatment timing, assessing disease activity through swollen and painful joint evaluation, along with measurements of CRP or ESR. The use of DAS28 helps define disease activity levels and treatment response based on EULAR response criteria [19].
The aim of this study is to identify and analyze predictive factors associated with a positive response to RTX treatment in patients with RA. This study explores the correlation and the importance of easily assessable clinical variables in routine clinical practice in order to understand their impact on the RTX therapy outcome.
Material and methods
Study design and patient population
We conducted a retrospective case-control study aimed at investigating the available clinical variables in routine clinical practice that predict the response to RTX therapy in patients with RA. The study population included seventy patients with RA who were treated with RTX at the University Clinic for Rheumatology in North Macedonia in the period between 2018 and 2023. All patients had active disease at the treatment onset, defined by the disease activity score in DAS28, and had previously undergone unsuccessful therapy with at least one DMARD (conventional synthetic or biologic). Each patient was treated with RTX doses of either 2 × 500 mg or 2 × 1,000 mg via intravenous infusion on days 1 and 15. Additional courses of therapy were administered at least 6 months afterwards, depending on the clinical response.
Data collection and assessment of disease activity
Patient information and treatment details were retrieved from the national database MojTermin. Baseline information collected at the time of RTX prescription included age, sex, disease duration, details of past and present antirheumatic therapies, disease activity evaluation (DAS28 score), Health Assessment Questionnaire (HAQ), ESR, CRP, and serological status for RF and ACPA. Concomitant DMARDs and present comorbidities were also recorded. Patients were followed up for 3 to 6 months for the DAS28 score, after their last RTX infusions. Those who were missing baseline information or follow-up data were excluded from this study. The primary objective of the study was to assess how patients responded to RTX treatment after 6 months, considering individual specific variables. A good response meant a significant decrease in DAS28 score (> 1.2), while no response meant the score decreased by ≤ 0.6 or 0.6–1.2 with a score of > 5.1. Responses in between were considered moderate. We divided our patients into two groups: responders to RTX therapy (moderate or good response) and non-respondents.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 26 statistical software. Descriptive statistics expressed sample characteristics as mean ± standard deviation (mean ±SD) for continuous variables and as numbers and percentages for categorical variables. Relationships between patient characteristics and therapy response were explored using Kendall’s τb and rank-biserial correlation coefficient, while ordinal logistic regression was used to predict therapy response. All tests were performed with a 95% confidence interval, and p-values less than 0.05 were deemed statistically significant.
Bioethical standards
Ethics committee of Faculty of Pharmacy, Ss. Cyril and Methodius University in Skopje, R.N. Macedonia agreed that the findings in this report were based on normal clinical practice and therefore were suitable for dissemination (02-284/4). All patients provided written informed consent at the time of study registration.
Results
The study group comprised 70 patients diagnosed with RA, including 57 women (81.4%) and 13 men (18.6%). The average age of the examined group was 56.3 years, with a standard deviation of 8.2 years. The youngest patient was 34 years old, and the oldest was 72 years old. A lack of response to therapy was observed in 23 patients (32.9%, non-responders), whereas a positive response was noted in 47 individuals (67.1%). Specifically, the response was moderate in 12 patients (17.1%) and good (indicating remission) in 35 patients (50%).
Table I displays the baseline patient and treatment characteristics for both the non-responder and responder groups. Among the patients who did not respond to the therapy, the majority were women (21 or 91.3%), while 2 were men (8.7%). The average age in this group was 55.3 years, with a standard deviation of 9.1 years. The youngest patient was 34 years old, and the oldest was 68 years old. The disease had lasted 9.7 years with a standard deviation of 6.3 years; the shortest lasted 2 and the longest 20 years. In this particular cohort, 15 patients (65.2%) exhibited high disease activity at the onset of treatment, while 7 (30.4%) showed moderate activity. The average CRP level in this group was 27.2 ±46.2 mg/l.
Table I
Patient and treatment characteristics
Among the patients who exhibited a positive response to the therapy, the majority were women (36 or 76.6%), and the average age within this group was 56.8 ±7.7 years. The youngest individual in this category was 40 years old, while the oldest was 72. The duration of the disease in this group was on average 11 ±7.1 years, ranging from 1 year to a maximum of 30 years in one patient. The most common disease activity level in this group was moderate intensity (25 or 53.2%), while the least prevalent were patients with moderate to highly active disease (4 or 8.5%). The mean value of DAS28 at the beginning of treatment was 5.3 ±1.0 ranging from 3.2 to 7.5, and that of CRP was 17.8 ±20.2 mg/l. In both groups, patients were categorized based on the degree of disability determined by the HAQ value: scores of 0 to 1 are generally considered to represent mild to moderate difficulty, 1 to 2 moderate to severe disability, and 2 to 3 severe to very severe disability.
In terms of additional RA treatments, the glucocorticosteroid (GC) prednisolone was the most frequently used medication, with 19 patients (82.6%) in the non-responder group and 35 patients (74.5%) in the responder group. The majority of patients had arterial hypertension (57.1%); among these, 12 (52.2%) were in the non-responder group, and 28 (59.6%) exhibited a positive response. Common comorbidities observed in both groups include osteoporosis (26.1% and 14.9% respectively), hypothyroidism (17.4% and 4.3%), hyperlipidemia (26.1% and 8.5%), and diabetes mellitus (13.0% and 10.6%).
The baseline serological status for RF and ACPA in both groups is detailed in Table II. Twenty-four percent (17/70) of patients did not show detectable levels for either RF or ACPAs. The group of patients who were either RF-positive or ACPA-positive showed a greater percentage of positive responses to therapy according to EULAR criteria at 6 months. The non-responder group showed a higher proportion of patients with seronegative RA, characterized by RF and ACPA negative results.
Table II
Rheumatoid factor (RF) and anti-cyclic citrullinated peptide antibody (ACPA) status
Table III outlines the correlations between patient characteristics and their response to treatment, while Table IV presents the results of ordinal logistic regression analysis investigating variables that may impact therapy response.
Table III
Correlations between characteristics of patients and their response to therapy
| Characteristic | Correlation coefficient | p-value |
|---|---|---|
| Sex | 0.215 | 0.073 |
| Age | 0.057 | 0.555 |
| Duration of disease | 0.12 | 0.214 |
| Disease activity | –0.328** | 0.003 |
| Rituximab dosage | –0.027 | 0.817 |
| DAS28 at baseline | –0.199* | 0.035 |
| DAS28 after 3 months | –0.444** | 0.000 |
| CRP | –0.051 | 0.622 |
| ACPA | 0.031 | 0.781 |
| RF | 0.067 | 0.485 |
| ESR | –0.247** | 0.009 |
| HAQ | 0.006 | 0.951 |
| Disability score | –0.032 | 0.773 |
| Additional therapy | – | > 0.05 |
| Comorbidities | – | > 0.05 |
Table IV
Ordinal logistic regression analysis of variables potentially influencing response to therapy
Discussion
The results indicated 4 significant associations among the clinical characteristics of patients who either demonstrated a positive response to EULAR criteria after 6 months of follow-up or did not. The first relates to persistence of disease activity (p = 0.003 and τb = –0.328), indicating that patients with persistently active disease were more likely to show a lack of response to RTX therapy. Patients with lower DAS28 values at the initiation of RTX treatment (p = 0.035 and τb = –0.199) and after 3 months (p = 0.000 and τb = –0.444) exhibited a more favorable response at 6 months. Patients with lower ESR values at the treatment onset achieved a superior response to therapy (p = 0.009 and τb = –0.247). All correlations are statistically significant at the 99% confidence interval. Our study found no significant correlations between responders and non-responses in terms of age, gender, disease duration, use of MTX and GCs, HAQ score, or CRP levels, which align with results reported in other comparable studies [11, 12].
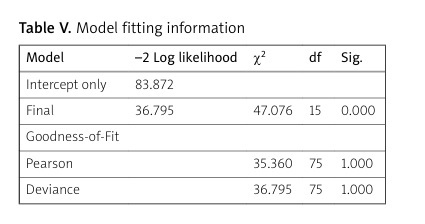
The main finding in our study is that the DAS28 value at treatment initiation exhibits the most significant predictive power in determining therapy response at 6 months based on assessed patient characteristics (p = 0.046). However, despite this, the multivariate analysis showed that the most effective model for predicting the EULAR response to RTX at 6 months included the interaction of all clinical variables examined in this study. The established regression model demonstrates adequacy (p = 0.000, χ2 = 47.076), and all the assessed characteristics are deemed suitable for predicting the therapy response in patients with RA (Table V).
Table V
Model fitting information
| Model | –2 Log likelihood | χ2 | df | Sig. |
|---|---|---|---|---|
| Intercept only | 83.872 | |||
| Final | 36.795 | 47.076 | 15 | 0.000 |
| Goodness-of-Fit | ||||
| Pearson | 35.360 | 75 | 1.000 | |
| Deviance | 36.795 | 75 | 1.000 |
In contrast, results from the multivariate analysis in another study showed that the best model for predicting a major EULAR response to RTX was composed of two variables: ACPA positivity and the number of previous anti-TNF agents used [12]. Despite the fact that the presence of RF and/or ACPA has been demonstrated to be associated with a more favorable treatment response in a number of studies [11–14], our study found no significant association between rheumatoid factor and anti-cyclic citrullinated peptide antibodies concerning therapy response.
In clinical practice, identifying factors that predict the response to RTX therapy aids in optimization of personalized treatment including planning the RTX re-treatment as well understanding the factors linked to treatment discontinuation. In this context, recent studies showed that concomitant use of two or more csDMARDs and concomitant use of GCs with RTX are factors significantly associated with extending the retreatment time interval [20]. Additionally, patients who were seronegative and who had previously failed other bDMARDs were more at risk of drug discontinuation [21].
Therefore, to enhance predictive precision and to optimize personalized RTX treatment approaches, it is essential to conduct further related investigations using predictive models involving larger sample sizes.
Conclusions
This study validates the effectiveness of the existing model for predicting treatment response, which involves the interaction of various factors such as age, initial disease activity, additional therapy for RA, sedimentation rate, serological biomarkers and HAQ score. Exclusion of any of the identified factors reduces the probability of achieving a more accurate prediction of the outcome of the RTX therapy 6 months after the start of the treatment. These findings contribute valuable insights for enhancing individual patient outcomes by identifying key factors that can guide treatment decisions in routine clinical practice, and serve as an initial step in developing advanced predictive models.