Introduction
Ankylosing spondylitis (AS), one of the most prevalent chronic autoinflammatory diseases from the spondyloarthritis (SpA) group, is associated with accelerated atherosclerosis and enhanced cardiovascular morbidity and mortality compared to the general population, which cannot be fully explained by the presence of traditional cardiovascular risk factors. To date, the mechanisms and mediators of increased non-traditional risk are not fully elucidated. Efforts to understand this phenomenon have often focused on the endothelium, which serves as an interface for multiple overlapping risk factors and has been proposed to represent an initial step in the pathogenesis and maintenance of all stages of atherogenesis – from plaque formation to plaque rupture and thrombogenesis. In this article we review the epidemiology, discuss the evidence for impaired endothelial function and potential mechanism controlling this state, and finally we summarize the data regarding the efficacy of drugs in reducing endothelial dysfunction in patients with AS.
Ankylosing spondylitis
Ankylosing spondylitis is a chronic, systemic, autoinflammatory disease of the spine and sacroiliac joints with variable involvement of peripheral joints and non-articular organs such as the eye, gastrointestinal tract, skin, heart, and vascular system. The onset of AS is most often during the third decade of life, more frequently in males than in females, and results in serious impairment of spinal mobility and physical function, which has an adverse impact on quality of life. The prevalence of AS is generally assessed to be between 0.1% and 1.4% globally [1]. Ankylosing spondylitis has a strong genetic predisposition – genetic risk factors contribute to 80–90% of the susceptibility to the disease, and the main risk factor is the presence of HLA-B27 antigen, an MHC class I molecule. Although the mechanisms of AS onset remain incompletely understood, all of the most often discussed hypothesis of the potential roles of HLA-B27 in the pathogenesis of AS lead to the abnormal production of proinflammatory cytokines – five of which are of particular interest in the initiation and perpetuation of inflammation in AS: TNF-α (which probably plays a key role in the generation and chronicity of systemic inflammation), IL-1, IL-6, IL-17, and IL-23 [2].
Epidemiology of cardiovascular events in ankylosing spondylitis
Standardized mortality ratios (SMRs) in patients with AS are higher than those in the general population (1.6–1.9), and often premature mortality is due to cardiovascular events [3–6]. In addition to higher SMRs, AS patients have an increased rate of cardiovascular morbidity [6–8].
Data on cardiovascular incidence in AS individuals are limited and heterogeneous. Several studies have shown that ischemic heart disease, cerebrovascular accidents and peripheral artery disease are more common in patients with AS than in the general population [9]. To date, most (but not all) studies have reported certain levels of increased risk for different cardiovascular diseases in AS patients compared with non-AS subjects. Eriksson et al. [9], in a cohort study, demonstrated that patients with AS are at a 30–50% enhanced risk of acute coronary, cerebrovascular, and thromboembolic events compared with the general population, which cannot be fully explained by traditional cardiovascular risk factors such as smoking, obesity, hyperlipidemia or dyslipidemia, arterial hypertension, and diabetes.
Assessment of endothelial function
In clinical trials endothelial function is typically assessed by measuring changes in vasomotor tone in response to various stimuli. The most widely used noninvasive method is flow-mediated dilation (FMD) – an ultrasound technique that measure changes in brachial artery diameter in response to shear stress-induced vasodilation, which is an endothelium-dependent process. In some studies, FMD has been demonstrated to correlate with biomarkers for subclinical atherosclerosis and future cardiovascular events, although there are a number of studies in which no such correlation has been found. One possible explanation of this inconsistency is the fact that FMD is a technically challenging method and may be influenced by many confounding variables – a negative correlation between the percentage flow mediated dilation and baseline artery size is recognized as a fundamental scaling problem leading to biased estimates [10]. Another challenge of FMD is variability across centers and the need for a well-trained and experienced operator to perform this examination [10].
Endothelial function could also be evaluated non-invasively by using laser Doppler imaging to measure microvascular perfusion in the cutaneous microcirculation – this technique has not yet been widely applied and validated in patients with AS. There have been some concerns that assessment of conduit artery function (assessed by FMD) is insufficient in reflecting what exactly is happening in the endothelium of microcirculation.
An expanding body of evidence demonstrates that microvascular dysfunction contributes to progression of target organ damage [11], and measuring microvascular vasodilation seems to be a promising research tool in individuals suffering from AS. It is worth mentioning that numerous molecules including adhesion molecules – intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), E-selectin, P-selectin; nitric oxide (NO); endothelin 1 (ET-1), asymmetric dimethylarginine (ADMA), an endogenous endothelial nitric oxide synthase (eNOS) inhibitor, inflammatory cytokines, regulators of thrombosis, and indicators of endothelial damage and repair – endothelial progenitor cells (EPCs), platelet microparticles, vaspin, and apelin have been proposed as biomarkers for endothelial dysfunction, but to date the clinical significance of most of these biomarkers remains uncertain.
To explain the relationship between indicators of vascular dysfunction, conduit artery stiffness and enhanced cardiovascular risk in AS patient markers of endothelial dysfunction are correlated with traditional cardiovascular risk factors (including hypertension, dyslipidemia, insulin resistance, diabetes mellitus, smoking, obesity), plasma markers of inflammation used in clinical practice (C-reactive protein – CRP, erythrocyte sedimentation rate – ESR), indicators of arterial stiffness (measured by pulse wave velocity – PWV, and aortic augmentation index – AIx), subclinical atherosclerosis examined by ultrasonography of carotid arteries (carotid intima-media thickness – cIMT), and AS disease activity scales (Ankylosing Spondylitis Disease Activity Score – ASDAS, Bath Ankylosing Spondylitis Functional Index – BASFI, and Bath Ankylosing Spondylitis Disease Activity Index – BASDAI).
Endothelial dysfunction
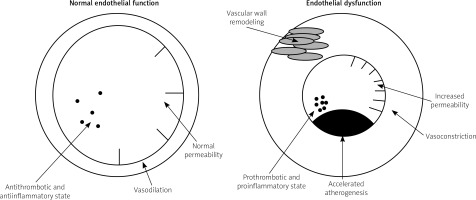
The endothelium – the inner lining of blood vessels – plays a critical and active role in the regulation of vascular tone and growth, smooth muscle migration, cellular adhesion and cellular interactions, thrombogenesis, thrombolysis, and maintenance of homeostasis between pro- and antithrombotic factors (Fig. 1). Impaired endothelial function is a state of endothelium which refers to its failure to cope with these physiological functions and is expressed as an upregulation of cellular adhesion molecules, compromised barrier function, increased vascular tone, and reduced resistance to thrombosis. In a number of studies endothelial dysfunction is restricted to endothelial vasomotor dysfunction and defined as an imbalance between vasodilating and vasoconstricting stimuli (both chemical and physical) acting on the endothelium or as an impaired ability of the artery to dilate in response to those stimuli, mainly caused by loss of endothelium-derived nitric oxide (NO) [12–14].
Because longstanding endothelial dysfunction promotes structural damage of the vascular wall and NO is one of the endothelial-derived factors with the strongest antiatherogenic effect, endothelial dysfunction is a major pathophysiological mechanism that leads towards the development of atherosclerotic cardiovascular disease [15]. Therefore, endothelial dysfunction represents a key step in the initiation, maintenance, and progression of atherosclerosis [16, 17] and may serve as an important marker responsible for atherosclerosis and future cardiovascular events [18]. Endothelial dysfunction has been reported to predate clinically obvious vascular pathology by years [19] and is also recognized as a predictor of adverse cardiovascular outcomes in the general population [20–22].
An increased prevalence of endothelial dysfunction in AS has been proven in several studies [23–25] along with the rising incidence of subclinical atherosclerosis [26]. Patients with AS often manifest endothelial dysfunction early in the course of the disease, which might be combined with traditional cardiovascular risk factors [27]; however, the association between inflammation, atherosclerosis, vascular dysfunction, and AS is multifactorial and still not entirely understood.
The link between endothelial dysfunction, atherosclerosis, and enhanced cardiovascular risk in patients with ankylosing spondylitis
Atherosclerosis is a chronic, progressive, inflammatory disease with a long asymptomatic phase. Mechanisms involved in systemic inflammation have been shown to contribute to atherosclerosis, which is the leading cause of enhanced cardiovascular risk and one of the most important causes of mortality and morbidity in the cardiovascular system [28]. Endothelial dysfunction is an early, initially reversible step in the development of atherosclerosis [29], and atherogenesis begins at sites of endothelial injury. What is more, impaired endothelial function and inflammation per se play a role in all stages of atherosclerosis – from plaque formation to instability and eventually plaque rupture [15].
A great number of proinflammatory molecules such as CRP, fibrinogen, and cytokines (TNF-α, IL-1, IL-6, IL-10, IL-17, IL-23) are involved in this process. Therefore, biomarkers of endothelial dysfunction and inflammation are used as surrogate markers of subclinical atherosclerosis [30, 31]. There is also a considerable rationale that inflammation per se can deteriorate lipid profile.
Disturbances in lipid levels, especially lowering of serum high-density lipoprotein cholesterol (HDLc) and increasing concentrations of triglycerides and low-density lipoproteins cholesterol (LDLc), has been proven to accelerate atherosclerosis. In several studies, reduction in HDLc was more frequently found in AS than in healthy controls [31]. This phenomenon might be at least partially responsible for enhanced cardiovascular risk in AS subjects. In addition, the current data indicate that vasa vasorum neovascularization is attenuated by inflammatory processes in the vascular wall and is strictly connected with accelerated atherogenesis [31].
Patients with as present an altered profile of different endogenous regulators of endothelial integrity and angiogenic factors such as ET-1, EPCs, proinflammatory cytokines, and adhesion molecules – biomarkers that offer an opportunity to study the relationship between the development of asymptomatic atherosclerosis and increased cardiovascular risk, and may help to identify high-risk individuals who may benefit from targeted therapy to prevent further cardiovascular events.
Markers of endothelial dysfunction in patients with ankylosing spondylitis
A number of biomarkers presumably involved in atherosclerosis in AS have been studied and are presented in Table I. A considerable number of studies have reported that asymmetric dimethylarginine (ADMA), an endogenous NO inhibitor, is significantly increased in the blood of AS patients [32–36] and contributes to higher cardiovascular risk [36]. In a study performed by Przepiera-Będzak et al. [37] serum endothelin 1 (ET-1) concentrations were lower in AS patients than in controls; however, Sari et al. [34] did not show any statistically significant difference in its levels. Endothelial progenitor cell (EPC) depletion in AS inversely correlated with disease duration, activity, and inflammation and positively correlated with endothelial dysfunction found in FMD and cIMT [38, 39].
Table I
Proposed biomarkers for endothelial dysfunction in ankylosing spondylitis
[i] AS – ankylosing spondylitis, ADMA – asymmetric dimethylarginine, NO – nitric oxide, ET-1 – endothelin 1, EPCs – endothelial progenitor cells, ICAM – intercellular adhesion molecule 1, VCAM – vascular cell adhesion molecule 1, IL-8 – interleukin 8, EMP – endothelial microparticles, PMP – platelet microparticles, PMC – platelet-monocyte complexes, sCD40L – soluble CD40L.
The levels of adhesion molecules ICAM and VCAM were positively correlated with predisposing factors for cardiovascular disease [40], and IL-8 has been shown to be strongly correlated with clinical markers of disease activity (ASDAI and BASDAS) in patients with AS who are naive for anti-TNF agents [40]. Wang et al. [41] found that in AS patients serum levels of vaspin were decreased and associated substantially with impaired endothelial function measured by FMD.
Circulating endothelial microparticles (EMP) and platelet microparticles (PMP), known to be indicators and mediators of vascular damage, were not significantly elevated in AS patients without classical cardiovascular factors, but were considerably downregulated in patients treated with TNF inhibitors compared with subjects treated with NSAIDs and healthy controls [42]. This finding suggests that anti-TNF therapy may have a beneficial effect on endothelium homeostasis in AS. However, Orüm et al. [43] detected platelet activation reflected by high levels of platelet-monocyte complexes (PMC) and soluble CD40L (sCD40L) in AS patients but did not show any improvement of platelet activation expressed as levels of PMC, platelet-neutrophil complexes, P-selectin (CD62P), soluble E-selectin, and sCD40L in AS patients after anti-TNF therapy. Impaired FMD, increased cIMT and PWV in AS subjects compared with healthy controls were found by Bodnár et al. [24].
A study conducted by Sari et al. [23] demonstrated significantly lower FMD but did not show a difference in IMT of the common carotid artery in AS patients in comparison with healthy individuals. Erre et al. [32] did not show any statistically significant differences between AS and controls with regard to FMD, cIMT, and PWV.
The effect of drugs
Increased cardiovascular risk in AS patients and the fact that endothelial dysfunction is reversible after a reduction in atherosclerotic risk factor by life-style modifications and pharmacological treatment [44] provide a strong rationale for early therapeutic intervention. A beneficial effect of infliximab on endothelial dysfunction in non-diabetic AS patients, manifested by a reduction in level of biomarkers of endothelial cell activation such as soluble E-selectin and soluble VCAM-1, was observed by Genre et al. [45]. Also, Syngle et al. [46] found that infliximab improves both endothelial dysfunction measured by FMD and inflammatory AS activity in previously anti-TNF-naive patients.
Endothelial dysfunction evaluated by laser Doppler fluxmetry after iontophoresis of acetylcholine and impaired capillary recruitment obtained by videomicroscopy improved significantly after etanercept, another TNF-inhibitor treatment [47]. Rosuvastatin was associated with an improvement in FMD, arterial stiffness, carotid plaque regression, inflammatory disease activity, and dyslipidemia [48, 49]. Spironolactone treatment was found to be associated with an improvement in FMD and inflammatory disease activity [50].
Conclusions
Patients with AS are at higher risk for cardiovascular morbidity and mortality than the general population. Accelerated atherogenesis represents the pathogenetic link between cardiovascular comorbidity and inflammatory rheumatic diseases. Growing evidence demonstrates that even in the absence of clinically evident atherosclerosis this enhanced cardiovascular risk is reflected early in measures of endothelial dysfunction. The endothelium seems to serve as an integrating platform of assessing cardiovascular risk, and there is a strong rationale to investigate its role and significance in this process.
This research offers a great opportunity to elucidate the relationship between the asymptomatic atherosclerosis and elevated cardiovascular risk and may help identify individuals with AS, who may benefit from targeted therapy to prevent clinically evident cardiovascular disease.