Introduction
Axial spondyloarthritis (axSpA) is a group of inflammatory rheumatic diseases affecting the axial skeleton and sacroiliac joints [1, 2]. The first symptoms develop before the age of 45 years, with a peak at 20–30 years of age. Its characteristic symptom is inflammatory spinal pain, also referred to as inflammatory back pain (IBP) [3]. In addition to the axial symptoms, peripheral arthritis, enthesitis, and numerous extra-articular manifestations, e.g. uveitis, skin psoriasis, and inflammatory bowel diseases, may sometimes develop (combination of axial and peripheral SpA) [4].
Two tools are mainly used worldwide to assess axSpA activity: Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Ankylosing Spondylitis Disease Activity Score (ASDAS) [5].
Ankylosing Spondylitis Disease Activity Score and Bath Ankylosing Spondylitis Disease Activity Index – definitions and values
The most frequently used indicator of the axSpA activity is the BASDAI. It is calculated on the basis of the patient’s answers to 6 questions scored on an analog scale from 0 to 10 (Table I) [6]. The questionnaire assesses the general level of fatigue, severity of pain in the neck, back, and hips, the severity of pain and/or swelling in other joints, the severity of discomfort in pressure-sensitive areas, the severity of stiffness upon waking up, and the duration of morning stiffness. The questionnaire refers to symptoms experienced during the week prior to evaluation. The formula for calculation of the BASDAI value is simple: the arithmetic mean of the sum of the values of the first 4 questions is added to the arithmetic mean of the values of the last 2 questions; the result < 4 denotes low disease activity and ≥ 4 means high disease activity; BASDAI = Q1 + Q2 + Q3 + Q4 + (Q5 + Q6): 2 [7]. The elements of BASDAI are presented in Table I.
Table I
BASDAI questionnaire components [6]
| Question (Q) No. | Questions about symptoms in the last week | Severity of symptoms on a 0–10 scale |
|---|---|---|
| 1 | How would you describe the overall level of fatigue? | |
| 2 | How would you describe the overall level of neck, back, or hip pain? | |
| 3 | How would you describe the overall level of pain and/or swelling in other joints? | |
| 4 | How would you describe the level of discomfort in an area tender to pressure? | |
| 5 | How would you describe the level of morning stiffness after waking up? | |
| 6* | How would you rate the duration of morning stiffness in minutes? |
The ASDAS is also a tool for assessment of the activity of axSpA. Currently, it is regarded as the most objective parameter in assessment of both the disease activity and the response to treatment. It combines parameters evaluated by the patient (3 questions about symptoms), a comprehensive assessment of the disease, and one of the indicators of inflammation (erythrocyte sedimentation rate [ESR] or C-reactive protein [CRP]) (Table II) [8]. However, the assessment using ASDAS-CRP is preferred over ASDAS-ESR.
Table II
The components of the Ankylosing Spondylitis Disease Activity Score (ASDAS) [8]
Three questions in the ASDAS questionnaire are the same as in the BASDAI tool, i.e. the severity of back, neck, and hip pain, the duration of morning stiffness, and the severity of pain and swelling in other joints. The symptoms are rated on an analog scale from 0 to 10, taking into account the severity of symptoms experienced in the past week. The method for calculating the ASDAS value is complicated; hence, special calculators are commonly used for this purpose. The ASDAS-CRP formula is as follows:
ASDAS-CRP = 0.12 × Back Pain + 0.06 × Duration of Morning Stiffness + 0.11 × Patient Global + 0.07 × Peripheral Pain/Swelling + 0.58 × Ln (CRP + 1).
The following ASDAS ranges are adopted: value < 1.3 – no disease activity, ≥ 1.3 and < 2.1 – moderate activity, ≥ 2.1 and < 3.5 – high activity, and ≥ 3.5 – very high activity [9].
The summarized components of ASDAS are presented in the Table II.
Ankylosing Spondylitis Disease Activity Score versus Bath Ankylosing Spondylitis Disease Activity Index – comparison of indicators
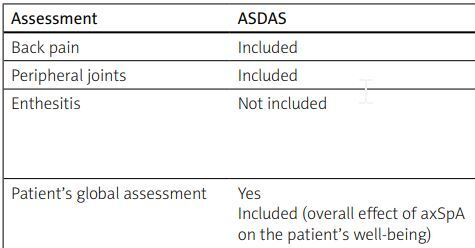
An important novelty and breakthrough in the ASAS/EULAR (Assessment in Spondyloarthritis International Society/European Alliance of Associations for Rheumatology) 2022 recommendations for the treatment of axial spondyloarthritis is the use of ASDAS as the preferred tool for assessment of disease activity to determine eligibility of patients for biological treatment or administration of Janus kinase inhibitors and to make decisions about continuation of therapy. Experts recommend the use of ASDAS in everyday practice, whereas BASDAI should only be applied when the ASDAS assessment is impossible [10] (Table III).
Table III
The comparison of ASDAS and BASDAI methods of assessment
| Assessment | ASDAS | BASDAI |
|---|---|---|
| Back pain | Included | Included |
| Peripheral joints | Included | Included |
| Enthesitis | Not included | Partially included (How would you describe the overall level of discomfort you have had from any areas tender to touch or pressure?) |
| Patient’s global assessment | Yes Included (overall effect of axSpA on the patient’s well-being) | Partially fatigue/tenderness level due to disease |
| Inflammatory biomarkers | Included | Not included |
| Patients’ self-assessment | Partially | Full |
| Comments/problem | Takes into account inflammation* | The patient’s responses may be associated with permanent disability (may lead to an error in the overall assessment) |
Recently, increasing numbers of studies have reported the superiority of ASDAS over BASDAI. In a retrospective study, Nam et al. found that a significant decrease in the ASDAS value after 3 months of anti-TNF (anti-tumor necrosis factor) treatment was a predictor of a good response to therapy with these drugs in the subsequent years of their administration [11]. Patients who responded to the treatment in agreement with the ASDAS assessment after 3 months showed improvement in chest expansion at 33 months of therapy and improvement in the BASMI value (Bath Ankylosing Spondylitis Metrology Index), i.e. a scale used to assess spinal mobility [11].
It has also been evidenced that the ASDAS value correlates with the level of inflammatory biomarkers, but this is not surprising considering that these parameters were included as part of the assessment. This scale additionally indicates relationships with the activity of osteoblasts. Similarly, relationships between ASDAS and metalloproteinase-3 (MMP-3), interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and osteocalcin have been identified. As indicated by ASDAS, significant improvement was associated with a greater percentage decrease in VEGF and MMP-3 levels and an increase in aggrecan content. In turn, a weak relationship was found between the BASDAI value and the concentration of IL-6 [12].
In comparison with other disease activity indicators, ASDAS exhibits the highest correlations with the severity of inflammatory changes in sacroiliac joints imaged by magnetic resonance imaging (MRI) [13]. A relationship between ASDAS and ADC (apparent diffusion coefficient), which is a radiological marker of axSpA disease activity in the MRI image, was also demonstrated, whereas no such relationship was observed between ADC and BASDAI [14]. Furthermore, a higher ASDAS value was correlated with a greater likelihood of formation of syndesmophytes [15], and their presence was found to increases the risk of formation of new syndesmophytes [16]. In turn, a low statistical correlation was found between BASDAI and the appearance of syndesmophytes in long-term observations [17].
A correlation was also observed between the ASDAS value and the risk of development of atherosclerosis. Increased arterial stiffness measured as augmentation index (Alx) was found at 5 years post diagnosis in patients with high baseline ASDAS values. However, high baseline BASDAI values were not associated with elevated AIx after 5 years [18].
In comparison with BASDAI, ASDAS is a better tool for discrimination of patients with high disease activity from those with disabilities caused by other factors and multiple comorbidities. It was reported that the BASDAI value was definitely overestimated in axSpA patients with concomitant fibromyalgia, while the ASDAS index represented the actual activity of axSpA [12]. A study conducted by Zhao et al. [19], who analyzed data provided by BSRBR-AS (the British Society for Rheumatology Biologics Register for AS), revealed a relationship between the BASDAI value and the number of comorbidities. The presence of subsequent comorbidities was associated with an increase in the BASDAI value by 0.4 units and by as much as 0.53 in the case of back pain. The ASDAS has also been recommended for use in AxSpA with comorbid inflammatory bowel disease or psoriasis to assess disease activity, in accordance with the 2022 ASAS/EULAR recommendations for treatment of axSpA [10]. In contrast, the comorbidities had no significant impact on the ASDAS value; hence, ASDAS can be regarded as a more objective tool [19].
In another study, conducted by Vastesaeger et al. [5], patients with axSpA were divided into three groups: with BASDAI ≥ 4, with ASDAS ≥ 2.1 (high ASDAS), and with ASDAS ≥ 3.5 (very high ASDAS). It was found in the study that the largest number of patients in the group with high and very high ASDAS values exhibited traits associated with a good response to anti-TNF therapy, i.e. a high CRP value, low degree/no enthesitis, male sex, and young age. The patients with very high ASDAS were younger, but they were more often diagnosed with enthesitis than those with elevated BASDAI values. In comparison with BASDAI, ASDAS turned out to be a better tool for identification of patients characterized by a positive response to anti-TNF treatment [5]. Another study proving the adequacy of the ASDAS tool for identification of patients who respond well to anti-TNF treatment was conducted by Marona et al. in a group of 594 axSpA patients from Portugal [20]. Eighty-two percent of the patients met the criteria for high activity of the disease on both the ASDAS (≥ 2.1) and BASDAI (≥ 4) scales. In comparison with patients meeting both criteria, those with only the high activity criterion according to ASDAS were more often males (77% vs. 51%); they were HLA B27 antigen positive (79% vs. 65%) and had an elevated CRP concentration [20].
In a Norwegian study conducted by Fagerli et al. [21] in a group of 289 axSpA patients, ASDAS ≥ 2.1 and BASDAI ≥ 4 were compared as eligibility criteria for starting anti-TNF treatment. The majority of patients (212) met both criteria, and this group achieved the best response to the treatment, whereas only 4 met exclusively the BASDAI criterion. More patients were qualified for the treatment using the ASDAS index than the BASDAI scale (260 vs. 216). It was also found that ASDAS performed satisfactorily in patients without elevated CRP and/or without peripheral joint swelling [21].
In a Taiwanese cohort study, Chen et al. [22] made an attempt to establish mutual cut-off values for BASDAI and ASDAS. In the case of ASDAS values of 1.3 (low disease activity), 2.1 (high disease activity), and 3.5 (very high disease activity), the corresponding BASDAI values were estimated at 2.1, 3.1, and 3.7 (all values < 4 indicating low disease activity according to BASDAI).
As shown by this study, if the BASDAI questionnaire is used exclusively, fewer patients will be deemed eligible for biological treatment, although this treatment is required. In conclusion, it was suggested that, since the BASDAI indicator is widely used worldwide, its value in determining eligibility for biological treatment should be reduced [22].
A study conducted by Nam et al. [23] in a group of 116 patients with ASDAS ≥ 2.1 and concurrently with BASDAI < 4 proved that, after 3 months of anti-TNF treatment, approximately 39% of the patients still had high ASDAS values (and simultaneously low BASDAI values), which may have resulted in higher risk of discontinuation of the therapy due to the low activity/lack of treatment efficacy indicated by the BASDAI assessment of the patients. The use of ASDAS alone instead of BASDAI or in addition to BASDAI may improve the assessment of axSpA patients. In turn, patients evaluated only with the BASDAI tool may not qualify for biological treatment or the treatment may be discontinued (BASDAI < 4) [23].
A Czech prospective study demonstrated that the ASDAS value correlated better with the risk of future disability than the BASDAI value. The ASDAS < 2.1 was a better predictor of low risk/no mobility disability in the future than BASDAI < 4 [24].
Given the recommendation to use mainly the ASDAS index in everyday medical practice, the ASDAS-CRP index was used to develop an ASDAS-Q index calculated based on qCRP tests. Blood for the qCRP test can be collected from the finger, and the result is available within 2 minutes. No collection of venous blood is required, and the agreement of the ASDAS disease activity index calculated with the qCRP test with ASDAS-CRP is 96%. Therefore, ASDAS-Q is regarded as a quick alternative to ASDAS-CRP [25].
The ASDAS does not differentiate between high CRP levels in comorbidities and high CRP levels in spinal inflammatory disease. Patients with high CRP levels in the course of an ASDAS infection will have very high CRP levels despite the absence of joint symptoms. The components of the ASDAS questionnaire are subjective and do not take into account clinician judgement, so comorbidities such as depression or fibromyalgia may falsely indicate high disease activity [12]. The ASDAS is not a perfect tool; the overall clinical condition of the patient, patient complaints, and chronic comorbidities or infections should be considered when assessing activity [19].
Conclusions
To sum up, as recommended by ASAS/EULAR 2022, ASDAS should be the preferred tool for assessment of the activity of axSpA and to determine patient eligibility to receive and continue biological treatment. If this is not possible, it should at least complement the BASDAI-based assessment. Disuse of ASDAS as a criterion of patient eligibility for biological treatment results in the exclusion of a large number of patients who could otherwise benefit from the treatment.
Ankylosing spondylitis disease activity score results are better correlated than BASDAI when the measures of disease activity assessed by patients and physicians are compared.
The ankylosing spondylitis disease activity score is a more reliable and objective tool for assessment of the activity of axSpA. It is not based exclusively on the patient’s response, and the information about the CRP level in the questionnaire increases its objectivity in comparison with BASDAI.
However, the investigator should individually exclude other causes of increased inflammatory parameters in the patient if they are to be taken into account in the assessment of axSpA activity using the ASDAS method.