Introduction
Pigmented villonodular synovitis (PVNS), or diffuse-type tenosynovial giant cell tumour (D-TGCT), is a rare disease of unknown origin affecting synovial tissue, with locally destructive effects on articular and para-articular structures.
Pigmented villonodular synovitis usually affects adolescents and middle-aged people, with women twice as likely to be affected. Some studies suggest females are slightly more predisposed, but only in the term of localized disease [1, 2], however, others suggest that male and female genders are affected at equal rates [3].
The disease typically has a monoarticular course, involving the knee joint (75%) or hip joint (15%), and less commonly other joints (ankle, wrist, elbow, or shoulder joints) [4].
Histopathologically, PVNS is characterized by synoviocyte proliferation and inflammatory infiltrations of lipophages and macrophages loaded with hemosiderin. Macroscopically, the prominent feature of the disease is the formation of nodules and villi of the synovial membrane of joints, bursae, and tendon sheaths.
Although histologically benign, these villonodular lesions can potentially infiltrate cartilage, bone and extraarticular structures, leading to functional impairment, disability, and, in extreme cases, necessity of limb amputation.
The primary mode of treatment is a surgical resection of the synovial tissue, either arthroscopically or by means of open, multi-compartment synovectomy [5]. The chances of long-term remission are increased when the surgery is followed with an adjuvant radiotherapy such as radionuclide synovectomy (RSV) or external beam radiotherapy (EBRT) [6]. Despite multi-modality treatment, the risk of recurrence is significant, ranging between 40 and 70% [7].
So far, there are no universally accepted systemic therapies that are effective in PVNS. Several publications suggest the tumour necrosis factor α (TNF-α) inhibitors might play a role in recurrent, treatment-refractory PVNS. Another, colony-stimulating factor 1 receptor (CSF-1R) and its ligands, CSF-1, regulate the function and survival of tumour-associated macrophages, which are involved in tumourigenesis and in the suppression of antitumour immunity. The colony-stimulating factor 1 receptor/CSF-1 axis has been implicated in the pathogenesis of pigmented villonodular synovitis (PVNS), a benign tumour of the synovium.
Material and methods
There are few data in the literature on the intra-articular treatment of PVNS. There are some reports of surgical treatment followed by an adjuvant radiotherapy [5, 6]. The relevant literature describing intra-articular treatment PVNS with TNF alfa inhibitors was reviewed.
A systematic search of the literature on the electronic database PubMed was conducted using the following combination of words: pigmented villonodular synovitis, combined with TNF alfa inhibitors (infliximab, etanercept, adalimumab, golimumab, certolizumab pegol) and intra-articular and treatment – between January 2005 and December 2020.
From the review of available literature in the last 15 years, only those directly describing TNF alfa inhibitor intra-articular treatment of PVNS were taken into account. Studies were also included if they evaluated not only surgical treatment but also RSV and either small molecules or antibodies in adult patients with PVNS. Articles describing PVNS treatment of children were not included.
The language of the chosen articles was restricted to English. The discussion was based on the case study and a literature review (Fig. 1).
Case description
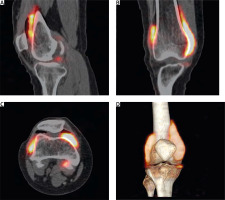
We present a case of a 29-year-old Caucasian female with histopathologically confirmed PVNS of the right knee joint. She underwent open surgical synovectomy followed with RSV (400 MBq of 90-yttrium citrate) with good initial results (Fig. 2).
Fig. 2
Positron emission tomography/computed tomography with 90Y-citrate after radiosynovectomy of the right knee joint (sagittal, coronal and transverse cross-sections, and a 3D volume rendering). Diffuse distribution of radiopharmaceutical in all compartments of the joint.
One year after the first-line treatment, the symptoms recurred, and the presence of villonodular lesions in the right knee joint was confirmed with ultrasound (US) and magnetic resonance imaging (MRI). The second RSV with 90-yttrium citrate was performed, inducing remission for another 15 months. After the third RSV, the treatment effects lasted only 6 months. At that point, another RSV was not recommended, and the patient did not consent to undergo surgical intervention. Since the possibilities of standard therapies were exhausted, an attempt of experimental treatment with intra-articular TNF-α inhibitor was made.
According to singular case-studies, the most common and safest TNF-α inhibitor used to treat PVNS was infliximab [8, 9], which made it the drug of choice for our patient.
After obtaining approval of the Ethics Committee and written consent of the patient, the standard diagnostics as before any other biological treatment was performed, i.e. complete blood count, biochemical markers of liver and kidney function, human immune deficiency and hepatitis B/C virus assays, chest plain radiograph, breast US, and pulmonological and gynaecological consultation.
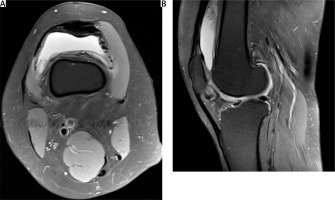
All the results were within normal limits, no contraindications for biological treatment were found. Right knee MRI was also performed, which showed pathological synovial hypertrophy and a fluid collection in the suprapatellar recess (Fig. 3). The infliximab (Remsima) was obtained as a donation from EGIS Polska.
Fig. 3
T2-wieghted fast spin-echo magnetic resonance imaging of the right knee joint. Effusion and synovial villi in the suprapatellar recess.
The patient was administered a total of 7 infliximab injections, according to the 0–1–3–6–12–15–month regimen. Each time, the right knee joint was punctured using an aseptic technique under direct ultrasonographic guidance and 100 mg of infliximab dissolved in 10 ml of physiological saline was injected. Three months after the last injection, a knee MRI was performed to assess the treatment effects.
Results
Throughout the treatment period and 3 months after last infliximab injection, no significant clinical improvement was observed. Oedema, tenderness, and restriction of motion of the knee joint persisted. During each of the joint punctures, the excess of pinkish synovial fluid was evacuated.
The final MRI revealed disease progression, with generalized synovial hypertrophy, effusion, and numerous loose bodies in the joint cavity. The patient was subsequently referred for surgical synovectomy and joint debridement.
The patient tolerated the treatment with infliximab very well. There were no adverse effects; of particular importance, there were no signs of septic arthritis or systemic infection.
Discussion
Pigmented villonodular synovitis has clinical and histopathological characteristics of both benign proliferative disorder and a chronic inflammatory process. In essence, it involves a polyclonal proliferation of activated synovial macrophages, which, through secretion of TNF-α and other cytokines, continuously sustain inflammation in the synovial membrane and stimulate its invasive growth.
Similarly to systemic inflammatory arthropathies, inhibition of TNF-α in PVNS potentially leads to a reduction of the synovial inflammation and resolution of clinical symptoms. However, the gold standard of treatment for pigmented villonodular synovitis has traditionally been surgical excision with total synovectomy of the affected joint, either with an open or arthroscopic approach [10].
While both methods have a similar reoccurrence rate of PVNS post-surgery, the arthroscopic procedure has consistently demonstrated better functional and range of motion outcomes for the patient [5, 10, 11].
Recent literature reports single case studies demonstrating good effects of PVNS treatment with intra-articular adalimumab, etanercept, and infliximab. Moreover, the analysis of 1046 intra-articular injections of various TNF-α inhibitors suggests a very high safety profile of such therapy [12].
Colony-stimulating factor 1 receptor (CSF-1R) and its ligands, CSF-1 and interleukin 34 (IL-34), regulate the function and survival of tumour-associated macrophages, which are involved in tumourigenesis and in the suppression of antitumour immunity. Targeting CSF-1R via either small molecules or antibodies has shown interesting results in vitro but limited antitumor activity in vivo, in solid tumours.
Concerning PVNS, clinical trials assessing CSF-1R inhibitors have revealed promising initial outcomes. Blocking CSF-1/CSF-1R signalling represents a promising immunotherapy approach and several new potential combination therapies for future clinical testing [13].
Research from the years 2005–2020 includes 8 case studies of PVNS patients treated with systemic or intra-articular TNF-α inhibitors (Table I).
Table I
Intra-articular therapy with tumour necrosis factor-α antagonists for severe diffuse pigmented villonodular synovitis: drug, dosing, and clinical efficacy
| Intra-articular therapy (No. of cases) | Study | Dosing and effect of treatment (if data available) |
|---|---|---|
| Infliximab (1) | Kroot et al., 2005 ] | INF dose: 5 mg/kg and the time between injections was no longer than 8 weeks (2, 6, 14, and 20 weeks later, and bimonthly thereafter up to 54 weeks); controlled the signs and symptoms |
| Etanercept (2) Etanercept (1) | Fiocco et al., 2006 [14] Zhao X. et al., 2014 [19] | 12.5 mg weekly IA-ETN injection for 4 weeks, followed by extended arthroscopic synovectomy and 25 mg IA-ETN injection for 4 weeks; marked remission IA-ETN injection; 1-year remission |
| Adalimumab (1) | Kobak, 2011 [15] | Significant clinical and radiological disease regression |
| Infliximab (3) | Praino et al., 2015 [9] | 6 injections of 100 mg infliximab within 12 months; complete remission |
Kroot et al. [8] reported a case of a patient showing a significant clinical improvement after intra-articular infliximab, along with a histopathologically confirmed decreased expression of TNF-α in the synovial membrane of the affected joint.
Fiocco at al. [14] described 2 patients with recurrent PVNS, successfully treated with intra-articular etanercept injections. The patients regained full range of motion in the treated joints, and US evaluation revealed reduction of thickness of the synovial tissue.
Similarly, Kobak [15] reported clinical and radiological improvement after intra-articular adalimumab in a patient who did not consent to surgical intervention.
Praino et al. [9] effectively treated 3 patients suffering from severe, refractory PVNS with intra-articular infliximab. During a 12-month observation, all the patients remained symptom-free, with decrease of synovial hypertrophy and joint effusion in imaging studies. No adverse effects of the treatment were observed.
In our case, we did not observe a satisfactory response to the treatment. In imaging studies there was marked disease progression. The unfavourable results might be attributed to inadequate dosing and the long intervals between injections. In the study by Kroot et al. [8], the infliximab dose was 5 mg/kg and the time between injections was no longer than 8 weeks.
However, Praino et al. [9] reported good results with treatment regimen similar to ours, i.e. 6 injections of 100 mg infliximab within 12 months.
To properly assess the effectiveness of intra-articular TNF-α inhibitors as an adjuvant treatment in recurrent PVNS, prospective studies are needed, with larger numbers of patients and longer observation periods.
Research from the years 2005–2020 also includes studies of PVNS patients treated with other monoclonal antibodies and tyrosine kinase inhibitors, considering a novel CSF-1 pathway.
Emactuzumab and PLX3397 deserve a mention [2]. Emactuzumab is a monoclonal antibody that binds directly to the CSF-1 receptor on the surface of macrophages, thereby reducing or eliminating the effects of inappropriate CSF-1 production.
In a recent phase I study, 26 of 28 patients with PVNS had an objective response to treatment with emactuzumab [2]. The CSF1R inhibitor PLX3397 (which acts by blocking the molecular endpoints of CSF-1) showed not only a well-tolerated side effect profile but also a clinical response in 52% of patients [6, 16].
Most recently, the use of pexidartinib, a CSF-1 receptor antagonist, was approved by the FDA in August 2019 for use in patients with extensive disease, who are unlikely to benefit from surgical intervention [17].
The continuous activation of macrophages in PVNS is attributed to the overexpression of the macrophage colony stimulating factor-1 (CSF1) due to a mutation in the CSF1 gene, and the resulting overstimulation of the CSF1 receptor (CSF1R). It was shown that this pathway can be non-specifically affected by tyrosine kinase inhibitors such as imatinib or nilotinib [18]. However, a more targeted therapy is possible [19].
Pexidartinib is a novel, recently registered small molecule and is the first systemic therapy to be approved for PVNS. While the initial results are encouraging, it is important to note that a phase III trial had an overall response rate of only 39% [17].
Cassier PA et al. [20] described 29 patients from 12 institutions in Europe, Australia, and the United States treated with imatinib mesylate (IM – CSF1R inhibitor). Symptomatic improvement was noted in 16 of 22 patients (73%) who were assessable for symptoms.
The authors concluded that the benefits of alleviating morbidity in patients with localized PVNS/TGCT must be balanced against the potential toxicity of chronic drug therapy.
Conclusions
The mainstay of current PVNS treatment is surgery with adjuvant radiotherapy. Despite combination therapy, the recurrence rate can be as high as 70%, which is far from satisfactory. It highlights the need for a conservative therapy that could sustain remission after initial invasive treatment. Although promising, it remains to be determined whether TNF-αblockade can influence long-term outcomes in PVNS patients.
Another potential answer to the PVNS issue may be targeted, molecular therapy.
So far, in patients with recurrent and treatment refractory PVNS, approved therapeutic options are limited and the optimal standard of care remains to be determined.