Introduction
Psoriatic arthritis (PsA) is a heterogeneous disease with various manifestations such as dactylitis, enthesitis, spondylitis, and skin involvement [1]. In addition to musculoskeletal and skin manifestations, PsA is associated with an increased frequency of metabolic syndrome and its consequences [2]. Furthermore, patients have an increased risk of permanent deformity, workforce loss, decreased quality of life, and increased mortality [3].
The assessment of disease activity is important to evaluate the current clinical situation of PsA patients as well as their response to therapy. Various measures of disease activity have been established for all manifestations individually or by combining such measures into a single scoring system [4]. A preliminary study has been undertaken to develop a more comprehensive disease activity instrument for psoriatic disease [5]. It is thought that composite indices can evaluate disease activity more comprehensively in PsA [6]. First, the International Group for Research in Psoriasis and Psoriatic Arthritis (GRAPPA) has proposed a composite system for the domains of peripheral arthritis, skin disease, spinal disease, enthesitis, and dactylitis with the intent of the grid informing decisions on treatment [7]. Although minimal disease activity (MDA) was first accepted by OMERACT as an appropriate activity measure for determining treatment goals in rheumatoid arthritis, it was also validated in PsA [8]. Furthermore, MDA was successfully used and found to be associated with remission in patients with PsA in previous studies [9, 10].
While most PsA patients use conventional synthetic disease-modifying antirheumatic drugs (cs-DMARD) such as methotrexate (MTX) or leflunomide (LF), up to 40% of patients require treatment with biological disease-modifying antirheumatic drugs (b-DMARD) [11]. Anti-tumor necrosis factor (anti-TNF) agents, including infliximab (IFX), etanercept (ETA), adalimumab (ADA), certolizumab (CZP) and golimumab (GLM), ustekinumab (IL-12/23 inhibitor), and IL-17 inhibitors such as secukinumab (SECU) have been standard treatments in patients with moderate-to-severe PsA who are unresponsive to cs-DMARDs [12]. Biological DMARDs have been found to be safe and effective in patients with PsA in many clinical trials [9, 13, 14].
In this study, we aimed to evaluate the MDA status and associated factors in patients with PsA in our tertiary referral clinic.
Material and methods
This cross-sectional study included patients who met the CASPAR classification criteria [15] and had at least 6 months of follow-up data between 2001 and 2021. Clinical data were collected from patient charts with standard forms. Patients were initially evaluated by an experienced rheumatologist (MB) cross-sectionally for MDA at their last visits, and a consensus decision was made with another attending rheumatologist (MI) if needed. Patients who met at least five of seven criteria (tender joint count [TJC] ≤ 1/68, swollen joint count [SJC] ≤ 1/66, psoriasis [PsO] area severity index [PASI] ≤ 1, Visual Analogue Scale [VAS] ≤ 15, patient global VAS (arthritis and PsO) ≤ 20, Health Assessment Questionnaire-Disability Index [HAQ-DI] ≤ 0.5, and enthesitis number ≤ 1) were considered to achieve MDA [16]. Psoriatic arthritis subtypes were evaluated in 6 subgroups: mono/oligoarthritis, isolated axial involvement, polyarthritis, distal interphalangeal (DIP) arthritis, arthritis mutilans, and mixed type (axial involvement with peripheral arthritis). Biological DMARD treatment was initiated in patients who did not respond and/or were intolerant to at least one cs-DMARD for at least 3 months. Patients using cs-DMARD for at least 3 months and TNF inhibitors (TNFi) for at least 6 months were evaluated for MDA in cs-DMARD and b-DMARD arms, respectively. Adalimumab, CZP, ETA, and GOL were grouped as subcutaneous (s.c.) TNFi. Secukinumab and ustekinumab could not be used in the study period due to insurance regulations in Turkey. Primary clinical inefficacy was defined as developing at least one of arthritis, dactylitis, or enthesitis under TNFi treatment for at least 3 months of follow-up. Secondary inefficacy was defined as the loss of treatment response after at least 6 months in TNFi treatment. Withdrawal of the drug due to severe or opportunistic infections, patient requests, intolerance, allergic reaction, or insurance problems were defined as “other reasons” for TNFi discontinuation. Severe infections were defined as those that cause death, hospitalization, or require intravenous antibiotics. Bioethical Committee of the Istanbul University approval and written informed patient consent were obtained for this study (date-number: 2020-1065).
Statistical analysis
In this study, version 21.0 of the program SPSS Statistics (IBM, Armonk, NY, USA) was used for statistical analysis of data. In descriptive statistics, discrete and continuous numerical variables were expressed as mean, ± standard deviation, or median (minimum-maximum). Student’s t-test and independent Student’s t-test were used for parametric variables showing normal distribution in univariate analysis. Categorical variables were expressed as number of cases and (%) and the χ2 test was used to compare variables with categorical parameters. The logistic regression method was performed in multivariate analysis of clinical parameters. Survival analysis was performed by the Kaplan-Meier method. Values of p < 0.05 were considered statistically significant.
Results
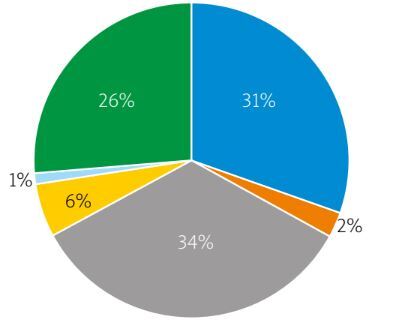
Data of 172 patients (61% female) were analyzed and included in the study. The flowchart of patients with PsA is shown in Figure 1. The median follow-up time was 98 (interquartile range [IQR]: 113, range: 6–444 months) and the mean age was 50.2 ±13.3 (range: 18–81) years. Mean age of onset for PsA was 38 ±12 (range: 11–79) years; median PsA and PsO durations were 144 (IQR: 114, range: 8–528) and 228 (IQR: 204, range: 0–756) months, respectively. The clinical characteristics of the patients are described in Table I and Figure 2. The median number of tender and swollen joints in patients before treatment was 4 (IQR: 5) and 2 (IQR: 3), respectively. While 96.5% of the patients received a cs-DMARD, MTX was the most common one (92%). Tumor necrosis factor inhibitors were used in 74 patients (43%); the most common first-line TNFi was ETA (n = 31; 42%) (Supplementary Table I).
Table I
Comparison of clinical and laboratory features of patients according to MDA status
| Variable | Total | MDA positive | MDA negative | p |
|---|---|---|---|---|
| Patient age [years], mean ±SD (range) | 50.2 ±13.3 (16–81) | 53 ±12.6 (27–81) | 46.7 ±13.6 (16–77) | 0.002 |
| Sex [n (%)] | ||||
| Male | 67 (39) | 39 (58.2) | 28 (41.8) | 0.6 |
| Female | 105 (61) | 56 (53.3) | 49 (46.7) | |
| Age at onset of PsA [years], mean ±SD (range) | 37.95 ±12 (11–79) | 40.2 ±11.3 (12–79) | 35.1 ±12.3 (11–64) | 0.007 |
| Follow-up time [months], median (IQR) | 98 (113) | 108 (98) | 72 (114) | 0.018* |
| Duration of PsA [months], median (IQR) | 144 (114) | 146 (113) | 118.5 (132) | 0.12 |
| Duration of PsO [months], median (IQR) | 228 (204) | 264 (204) | 192 (165) | 0.039* |
| Tender joint count (baseline), median (IQR) | 4 (5) | 4 (6) | 4 (4) | 0.4 |
| Swollen joint count (baseline), median (IQR) | 2 (3) | 2 (5) | 2 (2) | 0.2 |
| ESR [mm/h] (baseline), median (IQR) | 32 (34) | 31.5 (37) | 32 (28) | 0.96 |
| CRP [mg/l] (baseline), median (IQR) | 9 (17) | 7 (17) | 10.9 (16) | 0.18 |
| Tender joint count (after treatment), mean ±SD (range) | 1 ±1.9 (0–12) | 0.1 ±0.3 (0–1) | 2.2 ±2.4 (0–12) | < 0.001* |
| Swollen joint count (after treatment), mean ±SD (range) | 0.4 ±1.5 (0–12) | 0 (0) | 1 ±2.2 (0–12) | < 0.001* |
| ESR [mm/h] (after treatment) | 17 (18) | 13 (15) | 20 (19) | 0.14 |
| CRP [mg/l] (after treatment) | 4.8 (9) | 2.6 (5) | 11 (14) | < 0.001* |
| PsA subtypes [n (%)] | ||||
| Mono/oligoarthritis | 51 (29.7) | 24 (47.1) | 27 (52.9) | 0.6 |
| Polyarthritis | 57 (33.1) | 31 (54.4) | 26 (45.6) | 0.35 |
| Axial involvement | 4 (2.3) | 3 (75) | 1 (25) | 0.16 |
| Isolated DIP arthritis | 10 (5.8) | 7 (70) | 3 (30) | 0.07 |
| Arthritis mutilans | 1 (0.6) | 1 (100) | 0 | 0.16 |
| Mixed type | 44 (25.6) | 25 (56.8) | 19 (43.2) | 0.2 |
| cs-DMARD usage [n (%)] | 166 (96.5) | 91 (54.8) | 75 (45.2) | 0.6 |
| TNFi usage [n (%)] | 74 (43.3) | 34 (46) | 40 (54) | 0.038 (OR = 4.3) |
* Mann-Whitney U test. CRP – C-reactive protein, cs-DMARD – conventional disease-modifying antirheumatic drug, DIP – distal interphalangeal, ESR – erythrocyte sedimentation rate, IQR – interquartile range, MDA – minimal disease activity, PsA – psoriatic arthritis, PsO – psoriasis, TNFi – tumor necrosis factor inhibitors, SD – standard deviation.
Fig. 1
Flowchart of the analysis of patients with PsA.
MDA – minimal disease activity, TNFi – tumor necrosis factor inhibitor.
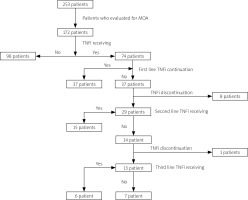
Overall, 95 (55.2%) of the patients were observed at MDA, which was lower in TNFi users compared to only cs-DMARD users (45.9% vs. 61.9%; p = 0.038, odds ratio [OR]: 4.3). Minimal disease activity did not differ either in PsA subtypes or among different TNFi. Moreover, the addition of cs-DMARD treatment did not affect MDA frequency in those who continued TNFi treatment (p = 0.87). In univariate analysis, MDA was associated with higher patient age (p = 0.002), longer PsO duration (p = 0.039), late onset of PsA (p = 0.007), and retention at first TNFi (p < 0.001, OR = 13.9) (Table II).
Table II
Treatment outcomes in patients who received TNFi treatment
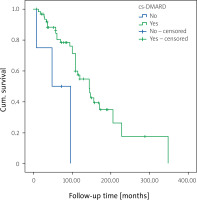
While 37 patients (50%) resumed the first TNFi, primary inefficacy was observed in 9 patients (24.3%), secondary inefficacy in 17 patients (46%), and treatment discontinuation in 11 patients (29.7%) due to other reasons. Twenty-nine patients switched to another TNFi treatment. Among those switchers, 15 patients (51.7%) resumed the second TNFi, 13 patients (44.8%) switched to third-line TNFi and 6 (46.2%) of these patients were still on third-line TNFi (Fig. 1 and Table II). The continuation rate of the first TNFi was significantly higher in males compared to females (p = 0.005; OR = 7.8). Tender and swollen joint counts at baseline were non-significantly higher in patients who retained the first-line TNFi (p = 0.052 and p = 0.06, respectively). Patient age, age of onset of PsA, duration of PsA and PsO, ESR and CRP levels at baseline, and PsA subtypes did not differ in terms of drug retention rate in univariate analysis (Supplementary Table II). Although concomitant use of cs-DMARD with the first TNFi did not differ in univariate analysis, the retention rate of the first TNFi was higher with concomitant cs-DMARD use (p = 0.008; Fig. 3).
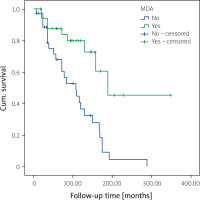
In multivariate analysis, higher patient age (B = 0.25, OR = 1.28, 95% confidence interval [CI]: 1.08–1.5, p = 0.004), late onset of PsA (B = 0.17, OR = 1.03, 95% CI: 1.01–1.067, p = 0.023) and higher retention rate of first TNFi (OR = 44.7, 95% CI: 4.6–434, p = 0.001) were associated with MDA (Table IV). Higher patient age (B = 0.26, OR = 1.3, 95% CI: 1.05–1.6, p = 0.016), male sex (OR = 29.3, 95% CI: 1.9–448, p = 0.015), higher age at onset of PsA (B = –0.24, OR = 0.78; 95% CI: 0.64–0.95, p = 0.0015), higher tender joint count at baseline (B = –0.46, OR = 0.63; 95% CI: 0.4–0.95, p = 0.027), and higher MDA frequency (OR = 335; 95% CI: 5.8–19,422, p = 0.005) were associated with higher retention rate of first TNFi (Table III and Fig. 4).
Table III
Multivariate analysis of factors associated with MDA and retention rate at first b-DMARD in patients with PsA
Discussion
In randomized controlled trials with TNFi, the proportion of patients achieving MDA is highly variable (24–52%) [17–19]. In the Swedish Early PsA Register, 40% of patients achieved MDA at the 5-year follow-up, following treatment with predominantly cs-DMARD or biologic therapies [20]. In a multicenter study from Turkey, external validation of MDA was performed [21]. A similar frequency of the patients had MDA status (42.5%) compared to low disease activity (45.7%) in the former study. In a prospective, multicenter web-based registry from Turkey, MDA was achieved in 46% of patients [22]. In our cohort, MDA frequency was compatible with the previous results. Minimal disease activity is a composite outcome measure that reflects the heterogeneous nature of PsA including musculoskeletal manifestations such as arthritis, dactylitis, and enthesitis as well as skin involvement, and also has stricter items than other outcome measures in PsA. Therefore, the relatively lower remission rate in MDA may be explained by the fact that MDA is a more comprehensive outcome measure than others. In a post-hoc analysis of the FUTURE 2 study, a higher proportion of patients with PsA could achieve DAPSA (Disease Activity in Psoriatic Arthritis) remission (DAPSA-REM) criteria compared to the MDA response, which is consistent with our findings [23]. Our study and other results indicate that although significant improvements have been observed in the treatment of PsA with TNFi in recent years, a higher proportion of patients could not achieve an MDA response yet. There is still an unmet need in the treatment of PsA to achieve higher MDA responses.
In our study, although MDA frequency was lower in patients using TNFi compared to cs-DMARD alone, it could be due to the initiation of TNFi in patients with higher disease activity at baseline, reflecting selection bias. In a cross-sectional analysis of a longitudinal cohort, MDA frequency did not differ between cs-DMARD and TNFi treatment [24]. In another observational, cross-sectional, multicenter study, the achievement of MDA was not significantly different between TNFi monotherapy, cs-DMARD monotherapy, or a combination of TNFi and cs-DMARD groups [25]. In our study, although MDA frequency did not differ with concomitant use of cs-DMARD with TNFi, a higher TNFi retention rate was observed in combination therapy. This may be due to the use of combination therapy in patients with relatively high disease activity at baseline.
In the literature, there are conflicting results that reveal the association of PsA treatment response and demographic characteristics. While no association was found between patient age and treatment outcome of PsA in the study of Mease et al. [9], younger patient age and male sex were associated with a higher MDA response rate in several observational studies [26–28]. Higher patient age and longer duration of PsA and PsO were found to be associated with higher MDA frequency in our study. Although male sex was associated with a higher MDA rate in previous studies [26, 27, 29], it did not differ in our study. On the other hand, higher TNFi retention was observed in males compared to females, in line with previous results [30, 31]. In a previous study, the presence of axial involvement and polyarthritis was associated with a lower likelihood of achieving MDA, while the presence of oligoarthritis was associated with a higher likelihood [32]. Minimal disease activity response was found to be associated with monoarthritis and negatively associated with DIP arthritis in another study from Turkey [22]. In our study, although it did not make a difference for MDA response, a higher retention rate of first TNFi was observed in patients with isolated axial involvement. There is limited evidence about axial involvement in PsA, and treatment responses of this manifestation in these patients were not well defined yet. Additional studies are needed on axial involvement and treatment outcomes in patients with PsA.
There are inconsistent results concerning the relationship between TNFi retention rate and baseline disease activity of PsA. Although there was no difference in one study [33], other studies revealed an association between high baseline disease activity and low retention rate on TNFi treatment [32, 34, 35]. In our study, higher tender and swollen joint counts reflecting the higher disease activity at baseline were observed in patients who resumed the first TNFi treatment, but it did not influence the MDA response.
Limitations
This study has some limitations. One of the main limitations of the study was the lack of disease activity score of PsA patients at baseline. Although the MDA response was evaluated cross-sectionally, the evaluation of patients’ treatment retrospectively was an important limitation and may have caused a bias in the treatment response. Comparison between TNFi could not be made due to the relatively small number of patients within TNFi subgroups in the second and third lines. The strength of our study was that it was a single-center study with a relatively high number of patients and a long follow-up period.
Conclusions
In our study, slightly more than half of the patients with PsA achieved MDA status. Although the frequency of MDA in our cohort was consistent with previous reports, a significant number of patients could not achieve MDA. A higher MDA rate was associated with a higher retention rate at first-line TNFi treatment and decreased gradually after TNFi switches. Minimal disease activity is a useful outcome measure to implement in daily follow-up of PsA patients, and the importance of reaching sustained MDA for prognosis should be investigated further.