Introduction
Nephrotic syndrome (NS) is a clinical condition characterized by daily loss of protein in the urine > 3.5 g/1.73 m²/day, hypoalbuminemia, hyperlipidemia, and the presence of edema. Most often, it is caused by primary glomerulonephritis (70%) [1]. In other patients, secondary glomerulopathies might be the cause of NS and are associated with [1]:
diabetes,
amyloidosis,
autoimmune diseases: systemic lupus erythematosus (SLE), systemic vasculitis,
infectious diseases: HIV, HBV, HCV, CMV, toxoplasmosis, parvovirus B19,
drug effects: gold salts, nonsteroidal anti-inflammatory drugs,
cancer,
renal vessel thrombosis: thrombotic microangiopathy, renal vein thrombosis.
Systemic lupus erythematosus is a chronic autoimmune disease that is associated with disturbances of acquired and innate immunity caused by various environmental factors and genetic predispositions. Lupus nephritis (LN) occurs in 35–75% of patients with SLE and manifests most commonly as glomerulonephritis [1, 2]. Proteinuria > 500 mg/day is the main symptom of LN. Also, NS may occur in the course of this disease. Lupus nephritis can also manifest as urinary changes such as granular casts, hematuria, and sterile leukocyturia, as well as acute kidney injury (AKI) or chronic kidney disease (CKD). In addition to clinical symptoms, kidney biopsy plays a key role in determining the diagnosis of LN. Based on the histopathological image, changes in kidneys can be classified into one of six classes (Table I) [3, 4].
Table I
Abbreviated International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification of lupus nephritis (2003, 2018) [3, 4]
| Class I | Minimal mesangial lupus nephritis |
| Class II | Mesangial proliferative lupus nephritis |
| Class III | Focal lupus nephritisa |
| Class IV | Diffuse segmental (IV–S) or global (IV–G) lupus nephritisb |
| Class V | Membranous lupus nephritisc |
| Class VI | Advanced sclerosing lupus nephritis |
| Indicate and grade (mild, moderate, severe) tubular atrophy, interstitial inflammation, and fibrosis, the severity of arteriosclerosis, or other vascular lesions |
Diabetic nephropathy is a functional and structural malfunctioning of the renal parenchyma due to chronic hyperglycemia. Diabetic nephropathy is a microangiopathic complication of diabetes and affects approximately 40% of diabetics. Microalbuminuria is an early symptom of diabetic nephropathy. 80% of patients with type 1 diabetes mellitus have overt proteinuria after 6–14 years of disease duration [5]. Diabetic kidney disease is currently the most common cause of end-stage renal disease (ESRD) [6]. Based on the histopathological image, diabetic nephropathy can be divided into four classes according to glomerular lesions (Table II) [7].
Table II
Glomerular classification of diabetic nephropathy [7]
Material and methods
As a background for discussion a case of nephropathy which was initially treated as a complication of poorly controlled long-lasting type 1 diabetes (DMT1) is presented. In the described patient apart from kidney involvement, no other signs of systemic connective tissue disease were observed. Diagnosis for autoimmune diseases was carried out due to the progressive nature of nephropathy despite constant diabetes treatment and an episode of thrombosis. The results of serological tests supported the diagnosis of systemic disease: SLE/APS.
Such a coincidence of events prompted the search for other descriptions and answers to the described clinical problem. Therefore the literature was analyzed using key words: diabetes mellitus type 1 and lupus erythematosus, nephrotic syndrome, lupus nephritis.
Case report
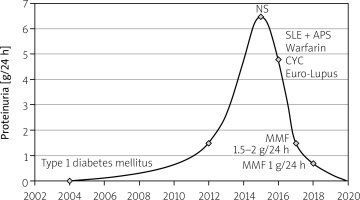
A 28-year-old female patient with type 1 diabetes was diagnosed at the age of 13 (2004) and at the age 22 (2012) with onset of proteinuria (1.5 g/day) and eGFR 90 ml/min/1.73 m2; therefore DMT1 treatment was started using a personal insulin pump. In 2015 the patient was admitted to the Endocrinology Clinic due to weight gain and massive swelling of lower limbs. Laboratory tests revealed microcytic anemia (Hb 10.2 g/dl), leukopenia (3790/ml) with lymphopenia (1000/ml), hypoproteinemia with dysproteinemia (total protein – 5.7 g/dl, albumin – 3.18 g/dl), proteinuria (6.5 g/day), and hypercholesterolemia. Renal function parameters remained normal.
Additionally, immunological studies showed a reduced concentration of complement components C3 and C4, antinuclear antibodies (ANA) presented in titer 1 : 2560 with speckled staining pattern, whereas pANCA and cANCA antibodies were absent. In abdominal ultrasound, the kidneys had normal size and structure. The patient also had an episode of deep vein thrombosis in the left upper limb.
At the age of 26 (2016), the patient was admitted to the Department of Rheumatology and Systemic Connective Tissue Diseases. The patient denied skin, mucosal and joint symptoms. The immunoassay tests were repeated: ANA 1 : 2560, speckled staining pattern, SS-A (++), SS-B (+++), Rib P (++) present in the ENA panel, anti-dsDNA borderline, anti-cardiolipin antibodies present in the IgM class – aCL-IgM (160.4 MPL; norm < 20 MPL) and in the IgG class – aCL-IgG (38.1 GPL; norm < 20), lupus anticoagulant was absent, but Factor V Leiden mutation was present.
Based on the clinical image and the results of additional tests, SLE was diagnosed. Hydroxychloroquine at a dose of 200 mg/day was recommended for treatment. Three months later, the anti-phospholipid antibody assay was repeated. High-aCL-IgM and aCL-IgG were detected, and anti-beta2-glycoprotein I antibodies in the IgM and IgG class (anti-b2-GPI IgM and IgG) were present.
Antiphospholipid syndrome (APS) was diagnosed, and warfarin anticoagulation was initiated. Due to the unclear etiology of nephropathy (diabetic? in the course of SLE? APS?), the patient was qualified for kidney biopsy. The pathomorphological image corresponded to class IV lupus nephropathy. Glucocorticosteroid and cyclophosphamide (CYC) pulses were used in the Euro-Lupus regimen. After 3 γ CYC administration, partial remission of lupus nephropathy was achieved: total protein and albumin levels were normal, and proteinuria decreased (3.2 g/day). Mycophenolate mofetil (MMF) at a dose of up to 2 g/day was recommended for maintenance of remission, hydroxychloroquine, and anticoagulant therapy were continued. After achieving partial remission, the MMF dose was reduced to 1 g/day. Since then, the patient has remained without clinical symptoms of SLE: proteinuria < 1 g/day, and eGFR 78 ml/min/1.73 m2 (Fig. 1).
Discussion
Analysis of secondary nephrotic syndrome causes
Nephrotic syndrome is a common symptom of autoimmune diseases, especially SLE. The percentage of patients with lupus nephritis who have NS ranges from 30% to 70%. The presence of NS significantly worsens the prognosis in patients with SLE [8]. Epidemiological studies indicate the coexistence of various autoimmune diseases in one patient, most commonly Hashimoto’s disease, type 1 diabetes, albinism, or inflammatory joint diseases. The co-occurrence of one or more autoimmune diseases in one patient increases the risk of damage to organs they have involved, including kidney damage. About 30% of patients with SLE develop another autoimmune disease [9].
Patients with SLE are at an increased risk of developing diabetes. The comorbidity of SLE and type 1 diabetes is relatively rare. In the Kota et al. study [10], SLE was diagnosed in 3 of 260 patients with type 1 diabetes. Cortes et al. [11] analyzed a group of 485 patients with SLE. Type 1 and type 2 diabetes were diagnosed in 3 (0.61%) and 6 (0.82%) patients with SLE, respectively. Some patients with type 1 diabetes with insulin resistance associated with insulin receptor antibodies are more likely to develop SLE. In these patients, a variety of antibodies are found, including SLE marker antibodies against dsDNA [11]. Anti-insulin antibodies were found in 5 out of 27 (19%) patients with SLE [12, 13].
In the group of metabolic diseases, the most common cause of NS is diabetes, especially long-term and poorly controlled. Many studies show the co-morbidity of diabetes and kidney disease, which is not a consequence of diabetes but another associated disease [14–17]. Both SLE and diabetes can lead to the development of NS and ESRD in a variety of mechanisms. The comorbidity of type 1 diabetes in patients with SLE significantly increases this risk [18, 19].
Renal involvement is associated both with primary and secondary APS. Antiphospholipid syndrome causes various pathophysiological effects concerning the kidneys [20, 21]. Antiphospholipid associated nephropathy (APSN), acute or chronic with nephritic or nephrotic syndrome, may be presented in kidney biopsy specimens as:
However, the renal biopsy was a decisive test that allowed the diagnosis of lupus nephropathy and the exclusion of thrombotic changes in the course of APS. Only intensive immunosuppressive treatment with cyclophosphamide made it possible to achieve NS remission, which was maintained by introducing MMF in long-lasting treatment. Performing kidney biopsies in patients with nephropathy and diabetes should be considered particularly in patients with a short duration of diabetes, presence of hematuria, NS, or another systemic disease [2, 14–17].
Conclusions
The comorbidity of SLE and diabetes and the symptoms of nephropathy can pose significant diagnostic difficulties and require a specific diagnostic approach. Differentiation of the underlying causes of kidney damage is crucial in undertaking the appropriate treatment. The nature of morphological changes in kidney biopsy may help in the diagnosis, classification of nephropathy, and further therapeutic decisions, which are different in diabetic nephropathy and lupus nephritis (diabetic kidney disease and lupus nephritis).