Introduction
Autoimmune polyendocrine syndromes (APSs), also called polyglandular autoimmune syndromes (PGASs), are a hereditary group of diseases. Blood tests show the presence of autoantibodies against antigens of certain endocrine glands; the organs also develop lymphocytic infiltrates. This autoimmunity causes progressive organ damage in the cytotoxic reaction, which is antibody-dependent; T lymphocytes are involved in this process. Currently APSs include the following syndromes: APS-1, APS-2, APS-3 and APS-4. Usually an organ affected by APSs becomes hypofunctional; however, the autoimmune process can manifest itself as hyperfunction, e.g. hyperthyroidism in Graves’ disease.
In the present article we focus on the connection between autoimmune polyendocrine syndromes and autoimmune rheumatic diseases as well as on management of patients with a combination of these clinical problems.
Autoimmune polyendocrine syndrome type 1
Autoimmune polyendocrine syndrome type 1 (APS-1), also called autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), or autoimmune polyendocrinopathy-1 (AP-1), is a rare monogenic autoimmune disease caused by loss-of-function mutations in the autoimmune regulator (AIRE) gene [1]. Autoimmune regulator deficiency impairs immune tolerance in the thymus and results in the peripheral escape of self-reactive T lymphocytes and the generation of several cytokine- and tissue antigen-targeted autoantibodies.
Autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy features a classic triad of characteristic clinical manifestations consisting of chronic mucocutaneous candidiasis, hypoparathyroidism, and primary adrenal insufficiency (Addison’s disease). Patients with APECED can develop other endocrine autoimmune manifestations, such as premature ovarian failure, testicular failure, autoimmune thyroid diseases (AITD), type 1 diabetes mellitus (T1DM), and lymphocytic hypophysitis. It is feasible that non-endocrine autoimmune disorders will develop, e.g. celiac disease, Addison-Biermer’s disease, autoimmune hepatitis, myasthenia gravis, alopecia areata, and vitiligo.
Autoimmune polyendocrine syndrome type 1 usually does not coexist with autoimmune rheumatic diseases. However, there have been a few single cases of articular disorders, e.g. childhood polyarthritis as an early manifestation of autoimmune polyendocrine syndrome with candidiasis and ectodermal dystrophy syndrome [2].
Autoimmune polyendocrine syndrome type 2
The APS-2 is associated with adrenal insufficiency (Addison’s disease), autoimmune thyroid diseases (Hashimoto’s thyroiditis or Graves’ disease), hypoparathyroidism, T1DM, hypogonadism, and hypopituitarism. The disease manifests itself in the 3rd–4th decades of life, and it is 5 times more common in women. The APS-2 is diagnosed in 10–20/100,000 people, and it is far more frequently identified in patients than APS-1.
Autoimmune polyendocrine syndrome 2 occurs often in siblings and parents and it is conditioned by polymorphism of numerous genes in major histocompatibility complex HLA class II. Prevalence of thyroid diseases and type 1 diabetes is associated with the presence of certain alleles of DRS antigens (DR3-DQ2) and DR4 (DR4-DQ8); in the case of Hashimoto’s thyroiditis (HT) it is DR3-DR5, and Addison’s disease is related to haplotypes DR3-DQ2/DRB, 0404-DQS. Recently, there have been some publications describing the significance of the following genes: CTLA-4, PTPN22, VDR, IL2RA, TNF-α, FOXP3, MICA, INS-VNTR [3].
Some environmental factors such as periods of stress or infections cause the first symptoms of the disease to emerge. In recent years it has been emphasized that people with sex hormone deficiency (hypogonadism) can develop an autoimmune disease [4]. Progesterone in women and testosterone in men are strong modulators of the immune response.
Autoimmune thyroid disease screening involves blood tests, such as thyroid stimulating hormone (TSH) and thyroid antibodies: anti-TPO (anti-thyroid peroxidase antibody) and anti-TG (antithyroglobulin antibodies), and in the case of hyperthyroidism symptoms also an anti-TSH test (anti-thyroid stimulating hormone receptor antibodies, also called TRAb) is conducted.
It is common that the symptoms of Addison’s disease manifest the earliest, but often they remain undiagnosed. In most cases AITD (Hashimoto’s thyroiditis) is recognized as the first one, due to widely accessible thyroid antibody tests. It is feasible that T1DM, premature ovarian failure, testicular failure (hypogonadism), hypoparathyroidism and lymphocytic hypophysitis may occur in the future.
It is frequent that patients with diagnosed Addison’s disease have the following antibodies: thyroid antibodies (anti-TPO and anti-TG together 50%), anti-parietal cell antibodies (APCA – 30%), anti-parathyroid antibodies (anti-PTH-a – 25%), ovarian antibodies (20%), testicular antibodies (5%), intrinsic factor antibodies (IF-a – 10%), islet cell antibodies (ICA – 5–10%) [5].
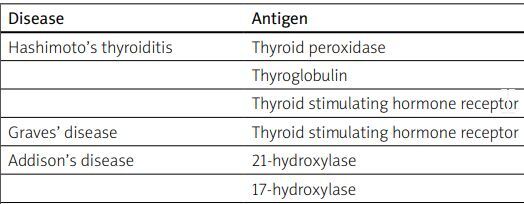
Serological diagnosis of APS-2 includes antibody tests against antigens of thyroid, adrenal glands, gonads, pancreas and intestines. It is essential to note that the antibody count may decrease due to the process of organ damage, which reduces the number of antibodies in the blood. Therefore, a lack of antibodies does not indicate the absence of disease (Table I).
Table I
Disorders in APS and antibodies
If T1DM coexists with Hashimoto’s thyroiditis, there is a risk of hypoglycemia due to decreased insulin demand. After restoring hormonal balance with levothyroxine in a patient with hypothyroidism, symptoms of hypoglycemia subside. There is a similar risk of hypoglycemia in patients with T1DM and Addison disease (autoimmune adrenal insufficiency); it is due to decreased glycogenolysis and increased insulin sensitivity. If patients develop Graves’ disease, their glucose tolerance becomes impaired (insulin tolerance becomes impaired too and glycogenesis in the liver is inhibited).
Treatment of numerous diseases constituting a part of APS-2 is not different from the one recommended for the treatment of the individual disorder. Adrenal hormone replacement therapy relies on administering hormones produced by 3 different layers of the adrenal cortex: cortisol in the zona fasciculata, fludrocortisone in the zona glomerulosa and dehydroepiandrosterone in the zona reticularis. The medication is taken orally: hydrocortisone in a daily dose of 20–25 mg (2/3 morning dose and 1/3 in the afternoon, replicating the circadian rhythm), fludrocortisone in a dose of 0.1 mg usually 2–3 times per week, dehydroepiandrosterone 25–50 mg in the morning. It is necessary to take the smallest but efficient doses of medication in order to avoid adverse effects. Patients should also be informed about the risk of acute adrenal crisis due to too low cortisol levels (decline in blood pressure and heart rate), and they ought to be made aware that they have to take a higher dose of cortisol (e.g., fever, planned surgery) [3].
Patients with hypothyroidism take levothyroxine in a dose of 1–1.5 μg/kg, checking the TSH levels every 4–6 weeks at the beginning. It should be noted that levothyroxine increases cortisol clearance; therefore, patients with undiagnosed adrenal insufficiency but diagnosed hypothyroidism and actively taking LT4 could experience an acute adrenal crisis.
It is possible to check the cortisol level in the morning (8:00 a.m.); it should be > 10 μg/dl.
Autoimmune polyendocrine syndrome type 3
Autoimmune polyendocrine syndrome 3 is considered to be a variant of APS-2. In order to diagnose APS-3 a patient needs to show symptoms of autoimmune thyroid disease without any symptoms of Addison’s disease. Autoimmune thyroid disease often coexists with type 1 diabetes (T1DM), premature ovarian insufficiency and rather rarely with lymphocytic hypophysitis [3].
Management of patients at risk of autoimmune polyendocrine syndrome
Management of patients at risk of autoimmune polyendocrine syndrome includes:
Monitoring of the patient with autoimmune rheumatic disease in order to be vigilant about possible coexisting APSs.
An interview and physical examination with special attention to APS symptoms.
Abnormalities in tests of the patients who show symptoms of APS.
Extension of diagnostics regarding APS and physical examination of the APS symptoms in patients with autoimmune rheumatic disease.
Autoimmune polyendocrine syndromes with rheumatoid arthritis (RA) may also be associated with celiac disease. Unsuccessful treatment with medication can be caused by an absorption disorder due to the damage of the intestine villi. Therefore, the patients show signs of vitamin, microelement and macroelement absorption issues, which can lead to muscle weakness, join infections and Raynaud’s disease.
Addison-Biermer’s disease, often connected with atrophic gastritis, causes a lack of vitamin B12 absorption in the ileum, which leads to megaloblastic anemia. Therefore, it is necessary to check blood cells in the peripheral blood cell count test (high mean corpuscular volume – MCV). Also, inflammation of the stomach lining can cause degeneration of the posterior and lateral columns of the spinal cord. The patients report sensations of tingling and numbness in the arms and legs, an electric shock-like feeling during forward bending of the neck, losing balance while walking, a metallic taste in the mouth and sometimes a fissured and enlarged tongue (Table II).
Table II
Diseases, symptoms and test abnormalities possible in APS
Autoimmune thyroid disease screening is advised especially to women with RA who are planning pregnancy or are currently pregnant. In a study conducted in 2017 thyroid peroxidase was found in granulosa cells of the cumulus oophorus in the ovarian follicle. For that reason, women with AITD are at higher risk of pregnancy loss and infertility [6] as anti-TPO antibodies have a pathogenic effect on the thyroid and ovaries tissue.
Discussion
Autoimmune polyendocrine syndromes are a group of autoimmune disorders affecting endocrine and non-endocrine organs and manifesting symptoms of at least two autoimmune endocrine diseases. Autoimmune rheumatic diseases such as RA, Sjögren’s syndrome (SS) and systemic lupus erythematosus (SLE) are the most frequent disorders occurring in the course of APS.
Rheumatoid arthritis
Autoimmune polyendocrine syndrome 3 involving autoimmune thyroid disease can also be comorbid with RA and myasthenia gravis. Liu et al. [7] conducted a meta-analysis of the 29 research papers available to May 2022. They examined 35,708 cases of patients in terms of possible coexistence of RA and thyroid disorders, confirming the high incidence of AID, especially hypothyroidism, in this group of patients. Bagherzadeh-Fard et al. [8] also found that there were twice as many cases of AITD coexistence with RA as in a control group.
Furthermore, Waldenlind et al. [9], who conducted a cohort study on a Swedish population of 8,090 people with RA and 80,782 without RA, also confirmed the frequent coexistence of RA and AITD. They claimed that the risk of developing AITD remained high in the period from 5 years prior to the RA diagnosis to 5 years after it and the highest peak was 12 months prior to the RA diagnosis. The authors advised taking into account a cause and effect relationship of RA and AITD especially in people genetically predisposed. It is still not clear why the risk of AITD decreases after RA diagnosis and the level of anti-thyroid antibodies drops in that group of patients. The authors believed that it was an effect of RA medication, e.g. TNF inhibitors reduced inflammation and at the same time they hampered AITD development [9].
The fact that AITD usually develops prior to RA leads to the conclusion that a detailed interview about possible symptoms of RA in people with AITD and, in some cases, RA diagnostic tests are necessary.
The connection between AITD and RA was also a subject of the analysis by Lazúrowá et al. [10], who identified a common possible genetic background of these two disorders. Having an autoimmune background, both AITD and RA are the effect of the interactions of environmental factors and genetic predispositions. As a result, a process of epigenetic changes starts, consequently autoimmune tolerance breaks and autoimmune disease develops.
There are numerous publications relating to the genetic predisposition to RA and also to AITD in the scientific literature. It is also notable that among genetic variations which predispose patients to certain diseases, there are common elements for these two disorders. They are listed in Table III.
Table III
Common genetic background in RA and AITD, type 1 diabetes and celiac disease
Cytotoxic T cell antigen 4 (CTLA4) is a protein encoded by the gene CTLA4. It is a costimulatory molecule of activated by antigen contact T lymphocytes and it inhibits further activity of T lymphocytes to prevent an overactive immune response. Abnormalities and polymorphism within the CTLA4 gene are related to genetic predisposition to AITD and RA. Mutations of that gene are present in other diseases that are considered to be a part of autoimmune polyendocrine syndromes, e.g. T1DM or celiac disease.
The PTPN22 gene encodes the protein phosphatase non-receptor type 22, also called lymphoid tyrosine phosphatase. The protein decreases T lymphocyte activity. Polymorphism within the PTPN22 gene is associated with a higher risk of genetic predisposition to both RA and AITD. Additionally, this gene mutation also correlates with a predisposition to T1DM, which can be a part of APS. A possible common genetic background of RA and other autoimmune polyendocrine syndromes justifies a detailed medical interview regarding APS in high-risk groups as well as, in some cases, extended diagnostics specific for individual disorders.
It was proved that women suffering from RA and with autoimmune hypothyroidism receive higher disease-severity scoring results in comparison to women without Hashimoto’s disease [11]. Furthermore, women with AITD and RA experience longer periods of morning stiffness than women without AITD. Some women with autoimmune thyroid disease have rheumatic symptoms even with correct function of the thyroid gland.
In most cases they experience pain of multiple joints, unclassified joint inflammation, mucus membrane dryness without fulfilling the criteria necessary for the diagnosis of SS, as well as pain and weakens of the muscles. Some of the joint dysfunction symptoms present in women with AITD are similar to symptoms typical of RA. However, if both disorders coexist, the disease severity becomes greater [10]; therefore, it can be assumed that anti-thyroid antibodies have a negative impact on RA.
Patients with RA, besides being likely to develop AITD 2–3 times more often, are also at greater risk of other disorders of autoimmune nature which are classified as autoimmune polyendocrine syndromes too, e.g. type 1 diabetes, or vitamin B12 deficiency.
It was also confirmed by the work of Emamifar et al. [12]. The authors claimed that 10.4% of RA patients and 5.8% of patients with vitamin B12 deficiency also experienced type 1 diabetes. However, most frequently RA coexisted with AITD – 11.8% of patients. The authors found that women with RA were more likely to develop AITD than men, and older RA patients had a higher type 1 diabetes incidence rate than the younger patients.
Numerous publications have reported more frequent coexistence of diabetes and RA [13, 14]. The authors believed that it was caused by the over-activated immune system as well as medication used in RA treatment. Tumor necrosis factor α (TNF-α), which plays a significant role in RA pathogenesis, is also a mediator of insulin resistance [15]. The analysis of Antohe et al. [16] showed that patients with RA who were treated with TNF-α inhibitors were less likely to develop T1DM; they also had reduced insulin resistance, and improved insulin sensitivity.
Additionally, the meta-analysis of Jiang et al. [13], which included 11 clinical and diagnostic studies and 8 cohort studies, confirmed the more frequent coexistence of diabetes in RA patients, especially T1DM. According to the authors, this disorder was 4.78 times more frequent among RA patients, whereas type 2 diabetes was only 1.41 times more frequent. Also, it was suggested by Ferraz-Amaro et al. [17] that impaired pancreatic beta cell function was to be blamed for the high frequency of diabetes in RA patients. Furthermore, insulin resistance was often observed in RA patients, especially in an active form of the disease with a difficult course [18].
On the other hand, the much higher incidence rate of T1DM in RA patients could be associated with a common genetic background and common environmental factors, which have a triggering effect. Both RA and T1DM have characteristics of polymorphism in the PTPN22 gene [3, 4]. Additionally, patients with RA who experience a severe inflammatory reaction also tend to have increased levels of TNF-α, which had a diabetogenic effect described earlier, and interleukin-6 (IL-6). This cytokine blocks insulin activity and it is considered as pro-diabetic [3, 4].
Westra et al. [19] also analyzed potential genetic variants connected with coexistence of RA with T1DM and indicated new directions for further research in their work. The variants presented by the authors are included in Table III.
Numerous publications have emphasized the frequent presence of autoimmune diseases comorbid with RA. Warjri et al. [20] described an RA patient who also suffered from celiac disease. Gluten-sensitive enteropathy is an effect of many factors: environmental, genetic and immune. Intestine related antigens could trigger overactivation of the immune system observed in many autoimmune diseases, including RA.
The scientific literature includes many examples of positive effects of dietetic interventions on the course of RA, which can confirm the role of intestinal antigens in triggering overactivation of the immune system. Podas et al. [21] suggested that RA is a disease which starts by activation of the immune reaction in intestines. This hypothesis was corroborated by the research results of Lupoli et al. [22], who stated that patients suffering from celiac and autoimmune thyroid disease are at risk of developing seronegative inflammatory arthritis.
Furthermore, Lerner et al. [23] stressed that there are numerous publications reporting patients with celiac disease who also have symptoms typical of RA, e.g. pain and stiffness in the morning, back pain, multiple joint pain, subclinical synovitis, and sacroiliitis.
There are also reports of the presence of loci which constitute a common ground for celiac disease and RA. Some of them are presented in Table III [23–25]. Products of the transcription of those genes participate in regulation of T lymphocyte activity, regulation of B lymphocyte proliferation and regulation of spermatocyte and adipocyte differentiation as well as Th1 lymphocyte differentiation. Besides common genetic factors, Lerner et al. [23] drew attention to medical history and Epstein-Barr virus (EBV), hepatitis C virus (HCV) and Mycobacterium tuberculosis (TB).
Autoimmune diseases comorbid with RA often remain undiagnosed, and in consequence they are left untreated. Dougados [24] and MacLean [25] emphasize that it is a significant issue because diseases coexisting with RA cause deterioration of human functioning, higher frequency of hospitalization and higher mortality. According to Dougados [26] and Jeong et al. [27] these are important socio-economic elements of human life.
A great deal of importance is given to the research, diagnostics and treatment of comorbid rheumatic disorders by EULAR (European League Against Rheumatism, now European Alliance of Associations for Rheumatology) [28]. Active research, diagnostics and treatments of autoimmune diseases coexisting with RA are also of great significance for the proper response to primary disease treatment. Emamifar et al. [12] reported that patients with AITD or other autoimmune disease do not respond as well to treatment of RA as patients who suffer only from RA.
This phenomenon may be related to a common genetic background. The shared susceptibility gene involved in the pathogenesis of RA and thyroid disorders is the HLA gene complex and is associated with anti-citrullinated protein antibodies (ACPA) positivity and therefore more aggressive disease. The higher percentage of ACPA ≥ 100 EU/ml in RA patients with thyroid disorders not only supports the role of genetic factors in the pathogenesis of thyroid disorders and RA, but also explains the poorer initial response to the RA treatment in these patients [12].
Sjögren’s syndrome and endocrine symptoms
Besides RA, it is possible that autoimmune polyendocrine syndromes or other autoimmune diseases can coexist with SS. Disorders such as vitiligo, SS and RA are related to HLA-DR4 and HLA-DR3 and HLA-DR2, which explains why patients with APS2 may manifest symptoms of those diseases [29].
Sjögren’s syndrome is relatively often classified as a part of autoimmune syndromes. The study of Rojas-Villarraga et al. [30] confirmed the high frequency of coexistence of numerous autoimmune syndromes in 1,083 patients of 4 autoimmune disease cohorts, and AITD comorbid with SS as the most frequent disorders in individual patients [30].
Sjögren’s syndrome is probably the most frequent disease of connective tissue associated with AITD, especially with Hashimoto’s disease. A few authors reported that full-blown SS may be even 10 times more frequent in AITD patients than in the general population [31].
Biro et al. [32] found that the frequency of SS in 426 patients with HT or GD was 17% and 5%. During more than 10 years of examination, Lazarus et al. [33] found that in a cohort of 114 patients with SS, c. 40% of patients had another autoimmune disease and AITD patients comprised the largest group (16%). The most common manifestation of AITD in SS was hypothyroidism. In most cases it was diagnosed before SS.
Similar results were obtained by Ramos-Casals et al. [34]: in a group of patients with SS 20% of the them had AITD and 16% had non-AITD. This confirms that more than 1/3 of the patients with SS also suffered from thyroid diseases, the most frequent manifestation of which was subclinical hypothyroidism.
Hashimoto’s coexistence with SS is relatively well documented. Additionally, it is interesting that both disorders share similar symptoms and even nonspecific anti-bodies (e.g. anti-nuclear antibodies, rheumatoid factor – RF). Symptoms such as keratoconjunctivitis and xerostomia are reported by as many as 30% of the patients with autoimmune thyroid disease. Positive anti-nuclear antibodies are present in as many as 20–55% of patients with AITD; therefore, they have to be monitored due to a high risk of development of other autoimmune diseases, including SS [35].
Moreover, Warfvinge et al. [36] described not only the resemblance of clinical symptoms but also functional tests and pathological changes in the biopsy of the lower lip and/or scintigraphy of the parotid glands in more than 50% of patients with AITD; 30% of them also had SS.
Due to such significant resemblance of the symptoms and significantly high incidence rate of AITD coexistence with SS, Amador-Patarroyo et al. [37] developed a hypothesis relating to the presence of common genetic, immunological and biological factors.
In the histopathological examination these disorders are characterized by the presence of similar tissue infiltrates consisting of mainly T CD4+ lymphocytes and activation of B lymphocytes [37]. Additionally, the researchers examined genetic features of SS and AITD by describing common HLA molecules expressed by thyroid and epithelial cells such as HLA-B8 and HLA-DR3 [37].
Furthermore, a strong pathogenetic relationship between AITD and SS is confirmed by the role of epithelial cells in the tissue inflammation and the presence of specific chemokines, such as CXCL10, which are described in AITD as inflammation markers causing tissue damage in SS. It was proved that epithelial cells make CXCL9 and CXCL10 and at the same time they contribute to the damage of salivary glands [38].
Additionally, a cytokine which plays a significant role in the pathogenesis of AITD and SS is B-cell activating factor. It seems to be especially important for autoreactive B-cells’ survival [39] and the concentration of it in the serum was described as much higher in Graves’ disease. If correlated with anti-thyroglobulin antibodies in the serum and with activity and severity of SS, it plays an important role in lymphoproliferative complications [40].
Data relating to the influence of AITD on SS are heterogeneous. Anaya et al. [41] when comparing a group of patients with Sjögren’s disease with a group with Sjögren’s and Hashimoto’s disease did not observe significant discrepancies in age, sex, and duration of the disease. The coexistence with Hashimoto’s disease did not have a major effect on SS with the exception of the presence of lymphadenopathy and urticaria. Zeher et al. [42] noted an average time gap between diagnosis of SS and Hashimoto’s disease which was 5.5 years and suggested that AITD was more often identified in people who have already suffered from SS.
Other authors believe that the patients with SS coexisting with Hashimoto’s disease can have less severe symptoms than patients suffering only from SS. Caramashi et al. [43] observed that people with SS and Hashimoto’s disease were less likely to be diagnosed with cryoglobulinemia, purpura, peripheral neuropathy, and a deficiency in complement C4 levels, which reduced the risk of lymphoma transformation. However, it is not clear if the diagnosis of SS comorbid with other disorders may increase the risk of lymphoproliferative complications in patients with AITD.
From the clinical point of view, due to the resemblance of symptoms and significantly high frequency of the two disorders’ coexistence, it is essential to perform screening tests for AITD on SS patients, and vice versa, regardless of the presence or absence of the symptoms, as it can impact the clinical picture and improve treatment results.
Among related autoimmune diseases, celiac disease and SS occur together with type 1 diabetes, autoimmune thyroid disease and primary cholangitis [44]. A large analysis of prevalence of celiac disease in equal groups of patients with SLE, SS, and systemic sclerosis showed a higher proportion of celiac disease compared to a control group of patients only with SS (6.8%) [45].
Celiac disease diagnosis usually occurs prior to SS. The age reported at the time of diagnosis among celiac patients who already had the syndrome was considerably higher than in people whose celiac disease was diagnosed for the first time. Interestingly, it was also found that prevalence of AITD in patients with Sjögren’s and celiac diseases was higher than in patients who only had SS.
On the other hand, Fasano et al. [46] recorded a low frequency of occurrence equal to 2% in a cohort of 98 patients with SS. A significantly higher rate (5.8%) in a group of 52 patients with SS was reported by Caio et al. [47], but the study was related mainly to serology without a histopathological confirmation.
A number of studies have focused on the spectrum of antibodies; the results showed a high frequency of antinuclear antibodies in patients with celiac disease ranging from 8.9% according to da Rosa Utiyama et al. [48] to 14.6% according to Erbasan et al. [49]. In the research analyzing the frequency of occurrence of antigliadin antibodies in equal groups of patients with diagnosed SS, RA and SLE, Teppo et al. [50] observed the highest levels of antigliadin antibodies as well as gluten and anti-reticulin antibodies in patients with SS.
The relationship between autoantibodies occurring in celiac disease and SS can be based on a common genetic background of these two pathologies, which is linked to DR3-DQ2 heterodimer encoded by the alleles DQA1*0501 and DQB1*0201 [51].
The correlation between SS and celiac disease seems to confirm a positive impact of gluten-free diet on autoimmune inflammatory process in salivary glands in patients with SS comorbid with celiac disease [52]. Interestingly, in another research work, Liden et al. [53] observed proctitis in patients with SS after a gluten challenge test.
It seems to be certain that celiac disease occurs more often in patients with SS than in the general population. There is insufficient evidence from a quantitative assessment of that relationship. Probably the coexistence of these two disorders is easily missed in clinical practice. It is necessary to test SS patients for celiac disease, especially if they are deficient in iron, or if they have hypertransaminasemia or elevated levels of pancreatic enzymes.
Sjögren’s syndrome is relatively often associated with other autoimmune diseases of autoimmune polyglandular syndromes, which was confirmed by numerous studies [33, 38]. Lazarus and Isenberg [33] noted that besides frequent coexistence of AITD, the following disorders were found: AIH in almost 3% of the patients, primary biliary cholangitis in c. 2% of the people and Addison-Biermer’s disease in almost 2% of the patients. Due to the resemblance of symptoms in patients with SS and in patients who are deficient in vitamin B12, i.e. chronic fatigue, mucosal changes or neuropathy, it is necessary to remember about the possible coexistence of those disorders and perform diagnostic tests.
Interestingly, Urbanski et al. [54] examining vitamin B12 deficiency found low levels of the vitamin in 43% of patients with SS and 11% in the control group. Moreover, the research work of Andrés et al. [ 55] showed vitamin B12 deficiency in 8.8% of patients with SS; however, there was no control group in that study, which does not allow one to draw a conclusion about the relationship between these two disorders. Besides case descriptions there are no publications on coexistence of SS and Addison-Biermer’s disease.
The APS-2 is defined as the coexistence of Addison’s disease with autoimmune disease and type 1 diabetes. Addison’s disease is a rare disease in comparison to AITD or myotonic dystrophy; however, as in the case of other autoimmune diseases, it often constitutes a part of other autoimmune polyendocrine syndromes. It is not known how frequently Addison’s disease coexists with connective tissue diseases, including SS. In the publications only descriptions of comorbid cases can be found [56].
Zellisen et al. [57] analyzed 91 patients with Addison’s disease and found the presence of other autoimmune diseases in almost 50% of the patients; the most frequent was AITD – 26.5%, followed by vitiligo – 9.7%, pernicious anemia – 4.8%, SS – 2.4% and several others.
Another study focused on the frequency of the occurrence of 21-hydroxylase antibodies, which are a marker of autoimmune adrenal insufficiency, in a cohort of 63 patients with SS. The results revealed the prevalence of autoimmune adrenal disease in nearly 20% of the people and its connection with markers of B lymphocyte activation [58]. Although the presence of antibodies was not associated with adrenal insufficiency, there was a worse response of adrenals in this group. Taking into consideration the fact that c. 50% of patients with 21-hydroxylase autoantibodies develop full-blown disease from 3 months to 11 years [59] it is necessary to monitor the patients. Patients with high levels of 21-hydroxylase autoantibodies are at an elevated risk of developing clinically evident adrenal insufficiency [ 60].
Patients with SS and a positive test for 21-hydroxylase autoantibodies complained about eye dryness less often than patients without 21-hydroxylase autoantibodies [61]. Owing to the better methods of identification of the new antibodies in SS, new opportunities are created, including new disease phenotypes’ identification and new aspects of its pathophysiology.
Systemic lupus erythematosus and autoimmune endocrine syndromes
Systemic lupus erythematosus, like SS and RA, can coexist with other endocrine and non-endocrine autoimmune diseases, which constitute a part of APS.
The presence of HLA-DR3 and HLA-DR4 haplotypes is common for SLE and APS-2. In spite of the fact that the haplotypes are not directly involved in the pathogenesis of the disease, they participate in antibody production, and thereby autoimmune phenomena [62].
In the study of McDonagh and Isenberg [63] up to 30% of patients with SLE diagnosis had at least one comorbid autoimmune disorder, of which the most frequent was SS – 13%, followed by RA – 6%, immune thrombocytopenia – 5%, antiphospholipid syndrome – 4%, Hashimoto’s disease – 4%, Graves’ disease – 2% and others. In most cases comorbid autoimmune disease occurs after SLE diagnosis (62%), which indicates a relatively young age of the patients developing SLE. Additionally, the SLE patients who also suffered from AITD were much younger in comparison to the patients who showed only SLE symptoms (32 vs. 41.5 years).
Among autoimmune endocrinopathies, SLE coexists with autoimmune thyroid disease (AITD) most frequently, which is, in fact, the most common autoimmune disease. A retrospective study of 218 patients with AITD showed the presence of another autoimmune disease – 13.7% of cases; the most common were SS and SLE [64].
Posselt et al. [65] observed that the frequency of Hashimoto’s disease in a group of patients with SLE was twice as high as in the control group (12.6% vs. 5.6%). The clinical picture showed that SLE and Hashimoto’s disease patients had a butterfly-shapes rash on the face less often; however, they had anti-Sm antibodies more often. It was not proved that there is a relationship between Hashimoto’s disease, SLE activity and organ damage in the course of SLE. Similar prevalence of Hashimoto’s disease of the SLE patients and a lack of a significant impact on the course of SLE were studied by Franco et al. [66]. A high incidence rate of comorbid thyroid disorders in SLE patients without any relationship with the clinical activity of SLE was described in other publications [67, 68].
It is often discussed whether SLE is an independent risk factor for these disorders, or it is incidental, because the group of patients at the highest risk of SLE consists of young and middle aged women; it is also true for autoimmune thyroid disease. Additionally, it is indicated that a common genetic background includes human leukocyte antigen (HLA-DR2, DR3 and DR8) [69]. A gene predisposing people to SLE (5q14.3–q.15) was also identified in AITD patients [70].
The most common changes in the thyroid of patients with SLE are clinical overt and subclinical hypothyroidism [71, 72], estimated at slightly more than 5%, 5 times more frequent than in the general population; on the other hand, hyperthyroidism (1.7%) was not significantly different [71]. Similar results were obtained by Miller et al. [73], who reported that the frequency of thyroid diseases in a group of SLE patients was 7.5%, including 6.6% of patients with hypothyroidism and 0.9% of patients with hyperthyroidism.
Besides high prevalence of thyroid diseases in patients with SLE compared to the general population, it is more frequent to detect antithyroid antibodies, both antithyroglobulin antibodies (anti-TG) and antithyroperoxidase antibodies (anti-TPO), without overt thyroid disease [72–73]. According to the study by Pyne and Isenberg [75], the incidence rate of thyroid antibodies ranged from 14% in the population with isolated SLE to 68% in patients with comorbid thyroid disease. The reason for that is not understood.
It is known that a single positive antithyroid antibody test has limited predictive value in patients with SLE because the antibody levels can fluctuate and even disappear completely, which was proved by some researchers [74]. According to Madagaro et al. [76] the fluctuations are caused by the change in the SLE activity; therefore antithyroid antibodies can be detected only in patients with an active form of SLE. There are also researchers who reported that the levels of TgAb, but not TPOAb, correlated with dsDNA levels in patients with lupus [77]. It is also known that SLE patients have numerous antibodies which are not present all the time, which still remains a subject of research.
Due to the frequent coexistence of SLE and thyroid diseases, in a case of unspecific symptoms in patients with SLE, especially when the activity of the disease is low, it is wise to consider the possibility of comorbid thyroid disease.
Patients with SLE, like patients with SS, are not at high risk of diabetes. Abugharbyeh et al. [78] analyzed the relationship between SLE, SS, systemic sclerosis and diabetes. The results show the inverted relations between the diseases, which suggest that they may have a different immunological pathogenesis. It is necessary to remember that there is a high risk of development of kidney and retinal diseases as well as peripheral neuropathy in patients with SLE and diabetes; therefore, detailed differential diagnostics are essential.
As far as Addison’s disease coexisting with SLE is concerned, there are only a few descriptions of such cases, which suggests that this combination is rare [79].
During the autoimmune inflammatory response, cytokines secreted from immune cells affect the endocrine system, which releases hormones which in turn modulate an immune response [80]. This confirms the interaction between immune, nervous and hormonal systems in modulation of the susceptibility as well as immunity to inflammatory diseases.
The main target of the activation of cytokines is the hypothalamic–pituitary–adrenal axis (HPA) [81]. It was proved that inflammatory cytokines such as IL-1a, IL-1b, IL-6 and TNF-α are strong activators of the HPA axis with release of a corticotrophin-releasing hormone (CRH) [82].
An inadequate response of the HPA axis to stress and chronic exposure to the stressor can be a triggering factor in autoimmunity [83]. It was observed that SS patients had lower levels of ACTH and cortisol compared to a healthy population, a low level of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) [84], as well as a weaker response of pituitary and adrenals to corticotrophin-releasing hormone stimulation (CRH) [85]. Similar abnormalities of the HPA axis were found in SLE [86], which seemed to promote chronic inflammation, and in patients with RA, who besides having very low levels of cortisol in a state of inflammation also had low levels of adrenal androgens [87].
Low levels of DHEA in serum are common in SLE and they correlate with the disease activity [88]. There were attempts to supplement DHEA in patients with SLE and in some trials the activity of the disease was reduced, including a reduction of glucocorticosteroids used so far [89]. Discrepancies in a stress response of the HPA axis may diversify patients who seem to have the same disease, but with varied responses to the treatment [90]. The HPA axis is significant in controlling the severity of SLE; however, disease development may change the HPA axis response.
In SLE and SS, besides hypofunction of the HPA axis, it seems that the hypothalamic–pituitary–gonadal axis (HPG) plays an important role too [91].
In the case of SLE, estrogens seems to be engaged in the pathogenesis of SLE [92]. Estrogens modulate the immune response [93], causing self-tolerance failure; they change the balance of Th1/Th2 in favor of Th2, induce T and B lymphocytes’ survival and autoantibody development with a secondary inflammatory response and organ damage [94].
Ovarian reserve is diminished even when symptoms of lupus are mild, which suggests a direct effect of the disease on ovarian function [95]. Manifestations of these abnormalities are irregular menstruations or a lack of them, and premature ovarian failure, which are relatively common in women with SLE, and many of them are connected with the disease activity [96]. Additionally, SLE induces hypothalamic–pituitary–ovarian axis dysfunction and increases the level of prolactin in the serum [97]. Prolactin also has an immunomodulatory function by being not only a hormone but also a cytokine, raised levels of which can lead to development or exacerbation of autoimmune diseases such as SLE [98].
In a study comparing levels of anti-Müllerian hormone (AMH) as a marker of the ovarian reserve of women with SLE and control group individuals, it was found that the patients with SLE had significantly lower levels of AMH than the control group women and it did not correlate with the activity of SLE [99]. These results show that SLE has a negative effect on the ovarian reserve and ovarian function.
What is known about the choice of treatment of rheumatoid arthritis (or another rheumatic diseases) in patients with APS?
Should the treatment of RA patients be different from the treatment of patients with RA and other autoimmune diseases? How to choose the best treatment? The effect of RA treatment on thyroid autoimmunity or other rheumatic diseases is little known. However, benefits of biological treatment, that is, TNF inhibitors to regulate and even depress thyroid autoimmunity, have been discussed recently, which delineates the possible role of TNF-α in a shared pathology between RA and thyroid autoimmunity [12].
A study on 138 consecutive adalimumab-treated patients with RA who were naive for TNF-blocking agents resulted in decreasing thyroid autoantibodies, that is, TPOAb and also TSH [99]. The authors concluded that TNF inhibitors could improve thyroid function in hypothyroid patients with RA. Furthermore, TNF inhibitors may regulate expression of proinflammatory cytokines and apoptosis in thyroids, leading to a reduced level of inflammation, earlier resolution, and decreased fibrosis.
Further investigations are still needed to enlighten the effect of TNF inhibitors on thyroid autoimmunity and other autoimmune endocrinopathies.
Conclusions
Taking into consideration the fact that the occurrence of autoimmune diseases coexisting with rheumatic disorders not only increases the risk of their development, but also affects treatment effects, the course of the disease and the quality of life and human functioning, an extremely important part of patient management is actively examining the patient for comorbid autoimmune diseases and proper treatment of them.
It will make it possible to ensure the comfort of the patients’ lives and optimize rheumatic treatment, and owing to the positive effects, it will result in greater satisfaction of the patients and their cooperation with a treating physician. It is extremely important, especially taking into account the fact that rheumatic diseases are associated with chronic pain and thus suffering of the affected ones. Therefore, improvement of patient physical wellness, fitness and functioning proportionally will be reflected in general physical and mental wellbeing of the patients.