Introduction
A new option in the management of severe rheumatoid arthritis (RA) is Janus kinase inhibitors (JAKi), which act on the signal transducer and activators of transcription (STAT) pathway.
The development of JAKi resulted from the discovery that the dysfunction in JAK leads to immunodeficiency in patients with rheumatic disease. The JAK-STAT pathway transmits signals from transmembrane receptors to the nucleus, controlling gene expression and regulating cell growth and differentiation through various cytokines, interferons, growth factors, and regulated molecules [1]. Janus kinase inhibitors modulate distinct cytokine pathways to varying degrees and duration over 24 hours.
A few decades ago, biological agents (biologics) improved the treatment of RA. These biologics target cytokines such as tumor necrosis factor (TNF), interleukin-6 (IL-6), and T or B cells. All biologic treatment options reduce the progression of joint damage in RA.
While complete recovery inflammatory rheumatic disease is not yet a realistic treatment goal, JAKi-STAT represent a modern treatment approach and have shown superiority over biologics. An important advantage of JAKi is their oral availability, compared to biologics, which require parental injection. All JAKi suppress the production of multiple pro-inflammatory cytokines within the cell, which is a significant advantage over biologics. For example, TNF inhibitors (TNFi) and IL-6 inhibitors only block a single cytokine.
However, JAKi may be used with safety considerations such as patient’s age (65 years or above), smoking, atherosclerotic cardiovascular disease, other cardiovascular risk factors, cancer risk factors, and risk factors for venous thromboembolic events (VTEs) and arterial thrombosis events (ATEs). These safety considerations are based on the results of the ORAL Surveillance trial [2], which indicated a higher incidence of these complications after treatment with tofacitinib (TOFA) in comparison with TNFi.
The aim of the study was to compare the effectiveness and side effects after treatment of biological disease modifying antirheumatic drugs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs).
Material and methods
Study population
This study is an expansion of the initial work published in 2022 in Reumatologia [3] with the current study including an analysis of patients treated with upadacitinib (UPA). The examinations were conducted between January 2010 and September 2021 on 130 patients (adults) with active definite severe RA, on the basis of American Rheumatism Association (ARA) criteria [4] and 2010 criteria [5], who were treated at the Rheumatologic Outpatients Department of the Central Clinical Hospital of the Ministry of the Interior and Administration, Warsaw, Poland. According to Larsen-Dale scores [6], all patients were in stages III–IV of the disease in radiographic assessment. Patients with other concomitant connective tissue diseases or contraindications to intended treatment were excluded from the study. Only patients with RA resistant to methotrexate (MTX) were included in groups treated with biologic agents or JAKi.
In the examined population, two groups were distinguished:
group I consisted of 80 patients treated with MTX 25 mg per week and with biologic agents;
group II consisted of 50 patients treated with MTX 25 mg per week and JAKi.
All patients were treated with a concomitant stable dose of glucocorticosteroids (GCS) – 10 mg of prednisone or less.
Clinical assessment
All patients underwent routine rheumatological examination regularly every 3 months of treatment for the number of tender and swollen joints out of 28 (10 proximal interphalangeal [PIP], 10 metacarpophalangeal [MCP], 2 wrists, 2 elbows, 2 shoulders and 2 knees) were recorded. The data on pain intensity on the Visual Analogue Scale (VAS) (0–100 mm) [7] were analyzed. Routine blood and urine tests were performed. From the obtained data, the Disease Activity Score for 28 joints based on the erythrocyte sedimentation rate (DAS28(ESR)) [8] and the Simplified Disease Activity Index (SDAI) [9] were calculated. Before treatment, X-rays of the hands and feet were taken to assess the initial degree of disease progression according to Larsen et al.’s criteria. The type and number of conventional synthetic DMARDs (csDMARDs), bDMARDs and tsDMARDs used to treat were assessed. Remission or low disease activity (LDA) was assessed by DAS28(ESR), SDAI and Boolean remission [10]. Switching between drugs was assessed. The frequency of adverse events was compared between the groups.
The ACR/EULAR Boolean remission is the following: tender joint count (TJC) ≤ 1, swollen joint count (SJC) ≤ 1, Patients’ Global Assessment (PtGA) ≤ 1 on a 0–10 scale and C-reactive protein (CRP) ≤ 1 mg/dl. In clinical trials, 61–66% of patients who achieved SDAI or clinical disease activity index (CDAI) remission also attained Boolean remission [10].
Statistical analysis
All results for categorical variables are expressed as counts and percentages, and Fisher’s exact test was used for comparison of proportions. Continuous variables are reported as mean ±SD (normally distributed data) or median and quartiles (Q1: 25th–Q2: 75th percentiles) in the case of skewed distribution. The differences between continuous variables were evaluated by Student’s t-test or nonparametric Mann-Whitney tests, as appropriate. Fisher’s test was applied to examine the homogeneity of variance. Within-group comparison was done using the paired Student’s t-test or Wilcoxon test. All hypotheses were two-tailed with a 0.05 type I error. All statistical analyses were performed using SAS 9.4.
Bioethical standards
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the Bioethical Committee of the District Medical Chamber in Bydgoszcz (consent No. 529/2012), District Medical Chamber in Gdansk (consent No. 667/2016), District Medical Chamber in Wroclaw (consent No. 21/05/2016). The consent of Eli Lilly to include the baricitinib (BARI) group in the presented analysis was obtained; the results of the Eli Lilly clinical trial have already been published. The consent of AbbVie to include the UPA group in the presented analysis was obtained; the results of the AbbVie clinical trial have already been published.
All patients provided written consent to participate in the study and authorization to use their health data.
Results
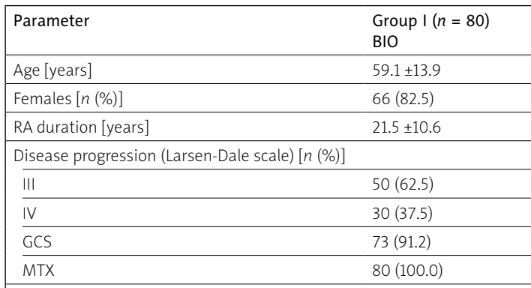
Table I shows baseline patient data before and after treatment. No statistically significant differences in patients in the two groups before treatment were found in such parameters as age, sex, RA progression and duration, number of tender and swollen joint, ESR, CRP, DAS28(ESR) and SDAI values. Patients treated with JAK-STAT inhibitors previously used a higher number of csDMARDs and bDMARDs than the group treated with bDMARDs. However, after treatment with a JAKi, this group of patients had lower values of SDAI (p = 0.046) and VAS (p < 0.001). Boolean remission was achieved in 28.75% of patients treated with biologics and 36% with JAKi.
Table I
Patients’ characteristics before and after treatment
[i] BIO – biologic agents, CRP – C-reactive protein, DAS28(ESR) – Disease Activity Score for 28 joints based on the erythrocyte sedimentation rate, ESR – erythrocyte sedimentation rate, GCs – glucocorticosteroids, JAKi-STAT – Janus kinase and signal transducer of activators of transcription, MTX – methotrexate, SDAI – Simple Disease Activity Index, VAS – Visual Analogue Scale.
Group I was described in detail in the previous study by Wisłowska [3].
In group II, JAKi were applied to 50 patients. Among the analyzed group, 24 used BARI, 22 UPA, 4 TOFA.
Methotrexate alone was ineffective in 35 patients, among whom 13 were on BARI and 22 on UPA. As the first therapy after MTX, UPA was used in all 22 patients. There were 15 cases of failure after MTX and biologics, as detailed in the previous paper [3]. Serious side effects were observed after TNFi in 4 patients, after infliximab (INF) infusion, allergy symptoms in 2 patients and lupus like syndrome in 1 patient, and after golimumab, breast cancer in 1 patient. Post-infusion allergy and syncope were observed in 2 patients with rituximab (RTX). Four complications were observed after tocilizumab (TOC): 1 herpes zoster meningitis and 3 cases of cancer (uterine body carcinoma – 1 case, thyroid gland carcinoma – 1 case, breast cancer – 1 case). Death due to cardiovascular complications occurred in 3 patients, 1 after INF, 1 after sequential INF and RTX and 1 after INF, etanercept (ETA), RTX. Serious side effects after BARI treatment were transient leukopenia (n = 1), skin basal cell carcinoma (n = 1), infection of left hip after alloplastic procedure after fracture and after TOFA infection of left hip. Additionally, 4 side effects were observed in cases of UPA treatment: transient rash on the skin (n = 1), skin basocellular carcinoma (n = 1), 2 patients had transient elevated D-dimer after SARS-CoV-2 infection.
Four patients treated with BARI were switched to TOFA; in 2 cases the treatment was changed to adalimumab (ADA) due to lack of improvement. In one patient, TOFA was discontinued due to colon perforation. Only 2 patients after TOFA and 2 after BARI did not achieve clinical remission, while after UPA all patients achieved clinical remission or LDA. Therefore, after BARI, patients were subsequently treated with RTX with good effect, and after TOFA and ADA were chosen with beneficial results.
The treatment effects in both groups were compared. The treatment was carried out in real life, and patients were in accordance with the clinical program and EULAR recommendations. If they achieved remission, they were still treated with a given drug; if not, they were treated with another drug. The results were similar, but the JAK-STAT group achieved lower SDAI and VAS values after treatment and more patients achieved Boolean remission (Table II).
Table II
Comparison of treatment effects in both groups
Discussion
Biological drugs have significantly improved RA patients’ quality of life. However, JAKi more significantly advanced the RA treatment by providing an effective oral therapeutic alternative to bDMARDs. These low-molecular-weight compounds block the activation of JAKs, which prevents the phosphorylation STAT proteins, critical regulators of inflammatory and immune responses. Janus kinase inhibitors offer the convenience of oral administration and improve patient-reported outcomes, especially those without improvement after treatment with MTX and bDMARDs.
Current therapies are not effective for all patients, and some may experience unacceptable side effects, necessitating multiple changes in medication. Additionally, good disease control will require personalization of therapy, which means tailoring treatment approaches to the individual needs of each patient.
Our current RA treatment goal is remission or LDA when remission cannot be achieved. The remission value using DAS28 based on the C-reactive protein level (DAS28(CRP)) is < 2.6, in CDAI ≤ 2.8, in SDAI ≤ 3.3. Remission according Boolean is when CRP values are ≤ 1 mg/dl and PtGA is ≤ 1 on a 0–10 scale [10]. According to the latest recommendations [11], if the goal of successful treatment is to be achieved, we start with MTX, the most frequently used DMARDs, both in monotherapy and combination therapy. It shows high efficacy and moderately low toxicity. When high disease activity is observed in RA patients despite MTX treatment, additional effects are sought through the use of biological drugs or JAKi. Among biologics, TNFi were the most commonly used initially [12–20], followed by the IL-6 receptor inhibitor TOC [21] and RTX, which targets CD20 on the surface of B lymphocytes [22]. The combination of MTX plus bDMARDs was superior in comparison with MTX alone in randomized, double-blind, placebo-controlled trials [12–22].
Regarding efficacy of treatment, its safety is particularly important. After biologics there may occur infections (bacterial sepsis, tuberculosis, atypical Mycobacterium, fungal infections, Pneumocystis jiroveci, Listeria monocytogenes), liver and hematologic pathologies, cancer, anaphylactic reaction, rash, or urticaria. After TNFi, additional side effects such as demyelinating syndromes, lupus like syndromes and worsening of cardiac heart failure have been observed. After TOC additional side effects include headache, elevations in total and LDL cholesterol, hypertension, and gastrointestinal perforation. After RTX side effects include infusion reactions, severe mucocutaneous reactions, hepatitis B virus reactivation (sometimes even fulminant hepatitis, hepatic failure, and even death), progressive multifocal leukoencephalopathy resulting in death, tumor lysis syndrome, cardiac arrhythmias and angina, bowel obstruction and perforation.
The efficacy of JAK-STAT inhibitors has been confirmed in many trials [23–25, 27–35, 36, 37]. Improvement occurs after 1 or 2 weeks to 3 months [23, 24, 26, 38]. The efficacy of JAKi vs. bDMARDs in MTX-resistant RA patients was confirmed: BARI 4 mg plus MTX was better than ADA plus MTX [25], UPA 15 mg plus MTX was better than ADA plus MTX [29, 31, 32], TOFA 5 mg [35] and filgotinib (FILGO) 100 mg/200 mg plus MTX were non-inferior to ADA plus MTX [33].
Janus kinase inhibitors’ safety as oral agents is due to their having the shortest half-life of any therapeutic class in rheumatology.
According to the ORAL Surveillance study [2], the post hoc analysis revealed a higher rate of major adverse cardiovascular events (MACE) connected with TOFA vs. TNFi in RA patients with atherosclerotic cardiovascular disease. This result affected the new recommendations for treatment of RA in the form of adding warnings about treatment with JAKi.
These inhibitors may be considered, but persistent risk factors (patients aged 65 years or above, smoking, atherosclerotic cardiovascular disease, malignancy risk factors, risk factors for venous thrombosis events) must be taken into account [11].
Other side effects during JAKi treatment are Herpes zoster reactivation, high-(HDL) and low-density lipoprotein (LDL) increase (the lipid profile is less atherogenic) and deep venous thromboses (DVTs)/(VTEs) [24, 26, 38].
Gastrointestinal perforations were observed less often after TNFi than after JAKi but most often after TOC. Leukopenia was observed during treatment with TOFA, UPA and neutropenia with BARI, FILGO, UPA [23, 24, 26]. Decreases in hemoglobin, although not clinically relevant, were also observed during treatment with UPA and BARI [24, 26]. Elevations in liver function tests were observed after using FILGO and UPA [26, 33]. Increases in creatine kinase and serum creatinine were observed in some patients during treatment with JAKi [24, 26].
Pawar et al. [39] reported occurrence of serious infections after JAKi vs. bDMARDs in a multidatabase cohort study. The hazard ratio (HR) for severe infection in 130,718 RA patients was as follows: TOFA vs. ETA 1.41, vs. abatacept 1.2 vs. TOC 1.17, vs. ADA 1.06 and lower than INF 0.81. Tofacitinib was associated with a 2-fold higher risk of Herpes zoster infection [39].
Upadacitinib and other JAKi may cause may cause fetal damage in pregnant women. Therefore, contraception is required during treatment with these drugs and 4 weeks after the last dose of the drug [24, 26]. Directions for new applications for JAKi are being sought; among others UPA is currently being evaluated in ongoing clinical trials for the treatment of giant cell arteritis, Takayasu arteritis, hidradenitis suppurativa, nonsegmental vitiligo, and systemic lupus erythematosus [41–44]. Currently a common situation is switching between JAKi and biologic agents and vice versa according to the last recommendations of treatment of RA [11].
In randomized studies, RA patients who did not show improvement after treatment with bDMARDs achieved clinical improvement after JAKi [25, 27, 29–32, 35]. However, there are few studies showing good effects of treatment with TNFi after ineffective treatment with JAKi [40].
Like these studies, the present study started similarly to others, with TNFi. In patients with a poor response to conventional or biologic DMARDs as well as with adverse effects of previous treatment, an attempt of JAKi treatment was made.
The value of Boolean remission is high, 28.75% in patients treated bDMARDs, and 36% in patients treated JAKi. More adverse events were observed after TOC and other JAKi.
JAKi are effective drugs in RA patients, convenient due to their short half-life. However, MACE, VTEs, ATEs, serious infections and malignancies must be taken into consideration before and during such treatment. For all the approved JAKi, mortality rates have generally been reported to be similar to those associated with bDMARDs including TNFi, with standard incidence ratios in the Surveillance [2], Epidemiology, and End Results (SEER) database of around 1 with no statistically significant difference. The Oral Surveillance trial raises more questions than it answers, but it does help to inform treatment decision-making for physicians and patients. The Oral Surveillance trial specifically enrolled patients 50 years of age with at least one cardiovascular risk factor to assess the relative risk of a JAKi vs. a TNFi. It was found, based on the statistical analysis, that TOFA was not non-inferior to a TNFi for the occurrence of MACEs and malignancy. This result does not mean that TOFA is inferior to TNFi, as there was a lack of statistically significant data to suggest that a TNFi might be superior to TOFA for MACEs and VTE.
Conclusions
Treatment with JAKi was successful, but the possible side effects suggest that the treatment may not be equally suitable for all patients. Treatment with JAKi is characterized by high efficacy, a convenient route of administration, and rapid reversibility, which is an advantage of this group of drugs.
Upadacitinib inhibits phosphorylation of downstream effector proteins, which consequently inhibits cytokine signaling for key pathways involved in inflammatory diseases. Upadacitinib met the primary end points and most secondary end points across all clinical trials and demonstrated superiority over placebo with standard of care background therapies in RA, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, and ulcerative colitis. The safety profile of UPA supported a favorable benefit–risk profile across all the approved indications.