Introduction
Rapidly destructive coxopathy (RDC), also known as rapidly progressive osteoarthritis (OA) of the hip, is a rare syndrome that can lead to swift destruction of the hip joint within 6 months to 3 years. First described by Postel in 1970, Lequesne shortly after defined the disease as chondrolysis > 2 mm within 1 year or a 50% reduction in joint space within 1 year, excluding other forms of destructive arthropathy such as avascular necrosis or Charcot neuropathy (CN) [1, 2].
The etiology of the disease remains unclear, while various pathophysiological mechanisms are presumed such as drug toxicity, autoimmune reactions, subchondral insufficiency fractures (SIF), etc. Although histological changes resemble those in primary coxarthrosis, the rapid disease progression and typical radiographic changes clearly differentiate rapidly progressive coxitis from OA [3]. The presence of chronic proliferative synovitis observed on ultrasound (US) and magnetic resonance imaging (MRI) is a remarkable feature of RDC, which appears to be useful in discriminating it from other forms of destructive arthropathies [4, 5]. The usage of US in the diagnostic work-up as a cost-efficient, fast and convenient modality has the potential to become a regular tool in assessing RDC patients.
Despite the efforts of many clinicians studying its etiology, epidemiology, pathogenesis and therapeutic approaches recently, RDC is still a mysterious condition with significant diagnostic difficulties and poor prognosis [6, 7]. In the literature, there are predominantly reports of isolated cases and case series, making the research of this condition even more difficult.
The aim of this case-based review is to provide a comprehensive analysis of a rare and poorly understood condition known as RDC, particularly in the context of its occurrence in a patient with rheumatoid arthritis (RA) and bilateral involvement of the hip joints. Through an in-depth examination of the presented case, we aim to elucidate the clinical manifestations, diagnostic challenges, potential etiological factors, and therapeutic considerations associated with RDC. Furthermore, we seek to highlight the importance of recognizing RDC as a distinct entity from other forms of destructive arthropathies, emphasizing the utility of imaging modalities such as US and MRI in its diagnosis. By addressing the gaps in the existing literature and sharing insights gained from our clinical experience, this review aims to contribute to a better understanding of RDC and facilitate improved management strategies for affected patients.
Search methodology and results
We utilized the recommendations outlined by Gasparyan et al. [8] to structure a case-based biomedical review systematically. In our pursuit to encompass all pertinent details, we conducted a thorough literature search spanning from the inception of PubMed (MEDLINE) and Scopus databases to October 20th and November 23rd, 2023, respectively. Using a comprehensive approach, we employed the Boolean search strategy, combining key words such as ‘rapidly’ AND (‘destructive’ OR ‘progressive’ OR ‘bilateral’) AND (‘coxopathy’ OR ‘hip arthropathy’ OR ‘osteoarthritis of the hip’). This meticulous search yielded 419 and 304 articles, respectively. Subsequently, through a rigorous process of eliminating duplicate articles, as well as excluding irrelevant articles, reviews, letters, editorials and conference papers, we identified original data for inclusion. Figure 1 provides a visual summary of our search methodology [9].
Our investigation yielded 26 articles suggesting a potential correlation between RDC and endocrine [10–13], metabolic [13–17], hematologic [18, 19], toxic [20–22], rheumatic conditions [23–30], or fractures/injuries [31–35] which we included in our analysis. Table I summarizes the clinical characteristics of patients with RDC associated with secondary conditions identified throughout the studies in the review, while Table II focuses specifically on RDC associated with RA.
Table I
Clinical characteristics of patients with rapidly destructive coxopathy (RDC) associated with secondary disease throughout the studies identified in the review
| Reports | Age [years] | Sex | Etiology/risk factors | Distribution | Imaging modality | Outcome |
|---|---|---|---|---|---|---|
| Huerfano et al. [10] | 76 | F | Diabetes mellitus | Unilateral | X-ray and MRI | Clinical improvement after surgical treatment |
| Nakamura et al. [11] | 90 | F | Osteoporosis | Unilateral | X-ray | N/A |
| Laroche et al. [12] | 67 | M | Chandler’s disease, diabetes mellitus | Unilateral | X-ray and MRI | N/A |
| Nakano et al. [13] | 61 | M | Amyloidosis, diabetes mellitus | Unilateral | X-ray and MRI | Significant clinical improvement after surgical treatment |
| Kitahara et al. [14] | 79 | M | Alkaptonuria | Unilateral | X-ray and MRI | Clinical improvement after surgical treatment |
| Araki et al. [15] | 56 | M | Alkaptonuria | Bilateral | X-ray | Clinical improvement after surgical treatment |
| Yang et al. [16] | 66 | M | Calcium hydroxyapatite deposition | Unilateral | X-ray and MRI | Clinical improvement after surgical treatment |
| Hamada et al. [17] | 69 | F | Alkaptonuria, subchondral insufficiency fracture | Bilateral | X-ray and MRI | N/A |
| Ishiguro et al. [18] | 48 | M | Hemophilia | Unilateral | X-ray and MRI | Clinical improvement after surgical treatment |
| Hama et al. [19] | 70 | F | Idiopathic thrombocytopenic purpura | Unilateral | X-ray | Clinical improvement after surgical treatment |
| Braaksma et al. [20] | 70 | M | Chronic alcoholic abuse | Bilateral | X-ray | Significant clinical improvement after surgical treatment |
| Tiwari et al. [21] | 62 | F | Intraarticular GC | Unilateral | X-ray | N/A |
| Xia et al. [22] | 65 | M | Alcoholic, osteoporosis | Bilateral | X-ray, CT and MRI | N/A |
| Lee et al. [23] | 37 | F | SLE, systemic GCs, alcohol and tobacco consumption, subchondral insufficiency fracture | Unilateral | X-ray and MRI | N/A |
| Yamamoto et al. [31] | 57 | F | Subchondral insufficiency fracture, intraarticular NSAIDs and corticosteroids | Bilateral | X-ray | N/A |
| Fukui et al. [32] | 77 | F | Subchondral insufficiency fracture | Unilateral | X-ray, CT and MRI | N/A |
| Nishida et al. [33] | 74 | F | Subchondral insufficiency fracture | Unilateral | X-ray and MRI | N/A |
| Watarai et al. [34] | 81 | F | Subchondral insufficiency fracture | Unilateral | X-ray and MRI | N/A |
| Kampani et al. [35] | 15 | M | HLA-B27 (+), chronic mechanical stress | Unilateral | X-ray and MRI | Significant clinical improvement after immunosuppressive and surgical treatment |
Table II
Clinical characteristics of patients with RA-associated RDC throughout the studies identified in the review
| Studies | Type of study | Patients included | Distribution | Imaging modality | Outcome |
|---|---|---|---|---|---|
| Yoshioka et al. [24] | Case report | 1 | Unilateral | X-ray, CT and MRI | Remission of RA after surgical treatment |
| Tateiwa et al. [25] | Case report | 1 | Unilateral | X-ray and MRI | N/A |
| Shu et al. [26] | Case report | 1 | Unilateral | X-ray, CT and MRI | Clinical improvement after immunosuppressive and surgical treatment |
| Matsumoto et al. [27] | Case report | 1 | Unilateral | X-ray and MRI | Clinical improvement after surgical treatment |
| Yoshino et al. [28] | Case series | 3 | All unilateral | X-ray and MRI | Clinical improvement after surgical treatment |
| Yun et al. [29] | Case series | 3 | All unilateral | X-ray, CT and MRI | Clinical improvement after surgical treatment |
| Kamiya et al. [30] | Case series | 2 | Bilateral | X-ray and MRI | Clinical improvement after surgical treatment |
The discussion section incorporates research articles and reviews that draw upon data from the cited references, diverse database inquiries, as well as the professional expertise, experience, and opinions of the authors. This collaborative effort forms the foundation of our narrative review.
For further literature analysis and discussion the case of an 82-year-old woman with a documented 11-year history of RA, who had been under the care of the Rheumatology Clinic at St. Marina University Hospital, Varna from 2011 to 2023 is presented. The diagnosis of RA was established in 2011 based on American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) classification criteria 2010 (symmetrical polyarthritis, high rheumatoid factor [RF] levels [> 130 IU/l], elevated C-reactive protein [CRP]).
The patient was notified that information related to the case would be submitted for publication, and their consent was obtained.
Treatment with methotrexate (MTX) 15 mg weekly was initiated. Follow-up in the clinic in 2013 showed a good response to MTX with normal acute-phase reactants: erythrocyte sedimentation rate (ESR) 30 mm/h, CRP 1.37 mg/l (< 5), RF 10 IU/l (< 12). During follow-up in 2016, significantly elevated acute-phase reactants and signs of anemia were noted due to discontinuation of MTX treatment, attributed to gastrointestinal adverse reactions (ESR 93 mm/h, CRP 100.3 mg/l, hemoglobin (Hb) 92 g/l, RF 114 IU). Treatment with leflunomide (LEF) was initiated. Follow-up in 2018 – the treatment with LEF was continued with moderate/low disease activity.
Currently the patient sought consultation with an outpatient rheumatologist due to difficulties in mobility, hip joint pain, as well as pain and swelling in the small joints of her hands and feet, coupled with morning stiffness lasting over 4 h.
Until 4 months prior to the patient’s last clinic visit, the patient had been managing adequately and independently. However, over the preceding four months she experienced a febrile episode accompanied by qualitative and quantitative consciousness disturbances, along with severe hip pain. During this period, the patient received treatment with intravenous ceftriaxone and ciprofloxacin antibiotics at home, administered by a general practitioner, effectively resolving the fever and consciousness issues. Nonetheless, the patient lost ability to move independently during this episode. Treatment with LEF was discontinued and not resumed during the febrile episode, and the patient does not currently take medications for any concurrent conditions.
On physical examination a generally compromised state was observed, responsive, and adequate with body mass index (BMI) 14.4. Visible mucous membranes were pale. The skin appearance was characteristic of glucocorticosteroid (GC) dermopathy. No deviations were observed in the respiratory, cardiovascular, gastrointestinal and urinary systems. Due to severe pain and restricted mobility, gait and stance could not be assessed. Examination of the musculoskeletal system revealed palpable tenderness in the carpal, metacarpophalangeal (MCP), and proximal interphalangeal (PIP) joints, shoulder, knee, hip, and ankle joints – Disease Activity Score with 28-joint count (DAS28) of 6.5. Soft tissue swelling in the wrists with severely limited range of motion was noted. Deformities characteristic of “rheumatoid hand” and “rheumatoid foot” were present. Crepitus was detected during active and passive flexion of the knee joints, and joint effusion was observed. External rotation of the femoral heads was preserved; however, active flexion was severely restricted. Painful but preserved passive ranges of motion were noted in all axes. There was a laboratory constellation for inflammatory activity: ESR 70 mm/h, CRP 12.27 mg/l, Hb 102 g/l, RF > 130 IU without any abnormalities in the biochemical assays.
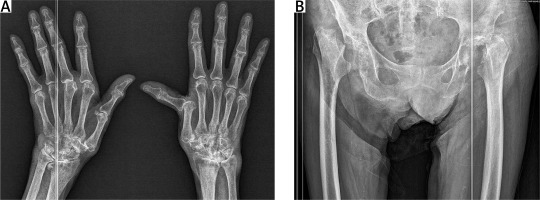
Conventional radiography of the hands was performed, detecting the presence of juxtaarticular osteoporosis, subchondral osteosclerosis, subluxation of the 2nd and 3rd MCP joints, and the 1st MCP of the right and left hand respectively (Fig. 2A). Joint space narrowing of PIP, MCP, and carpometacarpal joints was observed. Hip radiography indicated the absence of the head and cervix of the femur with cranial deviation of the metaphysis bilaterally (Fig. 2B).
Fig. 2
A) Rheumatoid hands showing periarticular osteoporosis, erosions, ulnar deviation of the fingers of the right hand with subluxation of the 2nd and 3rd metacarpophalangeal (MCP) joints, and the 1st MCP of the right and left hand, respectively. B) Hip radiography indicated the absence of the head and cervix of the femur with cranial deviation of the metaphysis bilaterally
Source: Radiology Department of University Hospital “St. Marina” – Varna, Bulgaria
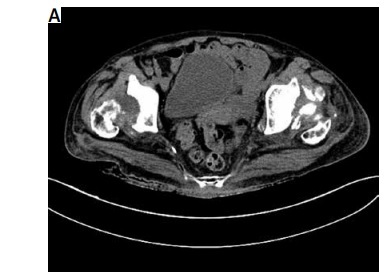
Heterogeneous dense opacifications were present in the acetabular fossae, suspicious of remnants of the femoral heads, which was confirmed by a computed tomography (CT) scan (Fig. 3A).
Fig. 3
A) Computed tomography scan showing heterogeneous dense opacifications present in the acetabular fossae suspicious of remnants of the femoral heads. B) Longitudinal scan of the anterior recess of the hip joint showing hip joint capsule distension with synovial hypertrophy and effusion. The absence of femoral head structure is due to destructive coxopathy in a patient with debilitating RA
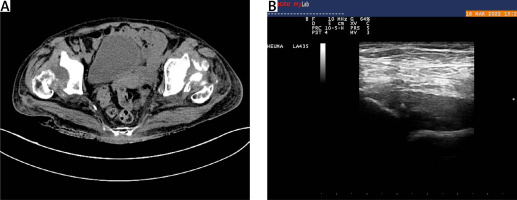
The size of the remnants measured was 28 mm and 24 mm for the right and left hip joint respectively. Bilateral superior-lateral luxation of the hip joints was observed. Ultrasound imaging confirmed the development of proliferative synovitis (Fig. 3B).
Several reasons for the rapid progression of arthritis were discussed, including avascular necrosis, old fractures of the femoral neck with subsequent bone resorption, aggressive course of RA, and destructive crystal arthropathy. Although septic arthritis is a prevalent cause for the development of destructive arthropathies, it was excluded from consideration in our case due to the simultaneous bilateral hip involvement, absence of characteristic clinical features of infection such as persistent fever and soft-tissue edema, as well as the lack of marked elevation in acute phase reactants and leukocytosis. Furthermore, upon thorough examination using various imaging modalities such as X-ray, CT scan, and US, no discernible signs indicative of infection were identified. Other discussed risk factors for the rapid and aggressive course of the condition include chronic low-dose systemic GCs intake and long-standing OA of the hip joints. Taking into consideration the high clinical activity of RA, the discontinuation of LEF, and their correlation with the systemic response and rapid deterioration of the hip joint, the diagnosis of RA-associated RDC was confirmed. It was decided that the patient is suitable for treatment with a biologic agent due to high disease activity and the lack of compliance to previous therapies. Treatment can commence after the surgical management of the hip joints.
Discussion
The etiology of RDC remains incompletely understood. In our review, we found a variety of endocrine, metabolic, hematologic, traumatic and rheumatic conditions associated with the development of RDC. Based on the gathered cases we identified potential risk factors for the development of bilateral RDC as chronic alcoholic abuse and the presence of alkaptonuria as a long-term comorbidity. Table I summarizes our results.
The pathophysiology of RDC is unclear. The role of synovitis and abnormal activation of giant multinucleated osteoclasts are discussed as they are found to be present in histology specimens from patients with RDC [36, 37]. Ogawa et al. [38] suggested that the activation of osteoclastogenesis occurred globally within the affected joint in RDC, rather than being limited to the vicinity of the destructive lesions. In our case, the long-standing RA in the context of sustained systemic GC intake and coexisting OA in an elderly female patient might be significant predisposing factors for bone metabolism dysregulation and accelerated hip joint destruction.
It is essential to consider additional research beyond the scope of our initial search. One pertinent case [39] provides valuable insights into the etiology and diagnostic challenges associated with RDC. While our case report and review addresses various factors contributing to RDC, including endocrine, metabolic, and rheumatic diseases, this specific case may elucidate the diagnostic complexities in distinguishing RDC from other rapidly destructive arthropathies.
Rapidly destructive coxopathy is diagnosed predominantly radiologically. Conventional radiography reveals joint space narrowing and the absence or minimal presence of osteophytes, and the presence of multiple subchondral cysts. Subsequent X-rays, typically acquired within a few months, illustrate the deterioration of both the femoral head and acetabulum [3]. Prominent characteristics observed in magnetic resonance imaging encompass a widespread pattern resembling bone marrow edema in the femoral head and neck, flattening of the femoral head, and the presence of cyst-like defects in the subchondral region. Supplementary observations consist of low-signal-intensity lines in the epiphysis, band-shaped regions exhibiting low signal intensity in the subchondral bone of the femoral head, and localized signal irregularities in the surrounding soft tissues, as depicted on short tau inversion recovery, fat-suppressed T2-weighted, or fat-suppressed gadolinium-enhanced T1-weighted MR images [4].
In our review, there were no cases or studies describing the usage of US imaging in the diagnostic work-up. We reported the presence of proliferative synovitis in an RDC patient on both hips by a US modality. Given the relatively early development of synovitis in RDC patients, we suggest the involvement of US imaging in patients with hip joint involvement and signs of swift progression as an alternative for early recognition of this condition.
Primarily the diagnostic criteria were defined by Lequesne as chondrolysis > 2 mm within 1 year or a 50% reduction in joint space within 1 year, with no evidence of other forms of destructive arthropathy [1]. Zazgyva et al. [5] proposed diagnostic criteria and staging for RDC based on the longevity of hip pain, limitation of joint mobility, the absence or reduction of osteophytes, and the presence of geodes in the femoral head or acetabulum. Nevertheless, there have not been established and confirmed standardized diagnostic criteria for RDC.
There are no specific laboratory markers for RDC. Synovial fluid analysis reveals bloody or neutrophil-rich sterile exudate, without the presence of crystals. Abe et al. [40] described elevated levels of interleukin-8 in synovial fluid in patients with RDC compared to OA, RA, and avascular necrosis. Masuhara et al. [41] found elevated levels of MMP-3 and MMP-9 from blood and synovial samples of patients with RDC. Several studies have investigated the potential role of bone turnover markers and osteoclast-related cytokines in both RDC and SIF. High concentrations were observed during the stage of bone destruction and collapse; however, they do not appear to highlight the early stages of disease development, making them unfeasible as early diagnostic markers in RDC [42, 43].
There is no established conservative therapy for RDC. Yasuda et al. [44] found that activation of STAT3 is present in the synovial tissues, particularly in synovial fibroblasts, during the initial phase of RDC accompanied by femoral head destruction and elevated serum MMP-3 levels. In their study administration of tofacitinib effectively inhibited STAT3 activation in synovial tissues affected by RDC, raising the suspicion of STAT3 emerging as a plausible therapeutic target for averting structural damage to joints in the context of RDC [44].
Strengths and limitations
The strengths of this study include a comprehensive search of the available literature with no language limitations, and the relatively complete information provided in the reviewed cases as well as the authors’ experience in the presented subject. However, it has several limitations. Firstly, hip MRI was not routinely performed, so we were not able to confirm any of the characteristic MRI findings and patterns described in RDC in the above-mentioned studies.
Secondly, we did not analyze an intraarticular aspiration of joint fluid in the literature or in the presented case description due to the absence of clinical, laboratory and imaging findings suggestive of septic arthritis or crystal deposition arthropathy. Finally, the main limitation of our analysis is the limited available literature and case reports regarding RA-associated RDC and the possible occurrence of selection bias.
Conclusions
Rapidly destructive coxopathy is a rare and challenging disease with unclear and multifactorial etiology and pathophysiology. The characteristic feature of progressive coxitis is rapid chondrolysis with minimal or no osteophytic formation.
Further research is needed to elucidate the underlying mechanisms and establish more effective diagnostic and therapeutic approaches for this challenging condition.
The fulminant nature of RDC, leading to rapid osteolysis of the femoral heads, highlights the importance of its early recognition and prompt diagnosis. The lack of definitive diagnostic criteria necessitates a high level of suspicion when encountering patients with hip joint involvement.
Additionally, the association with fever and cognitive disturbances, although atypical for coxitis, emphasizes the importance of considering systemic manifestations and a broad differential diagnosis in RA patients presenting with acute clinical deterioration.
The potential role of synovitis in the pathophysiology of RDC warrants further investigation, and the use of imaging modalities such as US and MRI may contribute to a better understanding of the disease mechanisms.