Introduction
Nowadays, thanks to medical advances an increased life expectancy may be observed. As a result, by 2050, more than 20% of people in society will be over 65 years old [1]. At present a healthy lifestyle based on physical activity is widely propagated. Unfortunately, this can lead to an increased number of injuries, especially in the above-mentioned age group. In terms of possible fractures, it is the proximal end of the femur, an area of their highest risk, which currently is a leading cause of hospitalization on trauma units [2]. These types of injuries are burdened with numerous complications, affecting patient mortality [3]. The fracture itself may be promoted by poor quality of bone structure. Nowadays, well-known and defined factors impairing bone tissue are osteoporosis, glucocorticosteroids, bone tumors and hormonal disorders [4, 5]. However, it has not been proven whether another factor predisposing to fracture is decreased muscle mass, especially considering age-related sarcopenia and the fact that the muscular system is responsible, among other things, for body balance, loss of which is one of the leading causes of falls and fractures [6, 7]. At present, the assessment of muscle tissue and bone quality necessitates carrying out dedicated and involving diagnostic tests [8, 9]. Gold standard measurement methods are costly, involve technical assistance and are associated with radiation exposure [10, 11]. Defining any biochemical factors indicating a patient’s higher predisposition to a proximal femur fracture (PFF) would be an easier and more readily available testing method. Therefore, the aim of our study was to define any relationship between muscle decay, determined by creatine kinase (CK), and PFF. Its significance in orthopedics is to date not fully explored, which creates a field for further research. Additionally, we decided to assess the bone quality in elderly patients on the basis of the C-terminal cross-linked telopeptide of type I collagen (CTX-I) and sex hormone binding globulin (SHBG) plasma levels. These proteins are proven to be useful markers reflecting bone remodeling and condition [12, 13]. We also evaluated the relation between CK and these recognized bone turnover markers.
Material and methods
The manuscript was prepared following STROBE guidelines.
In this observational study 40 consecutive patients were enrolled. They were all admitted to the 3rd level Medical University of Warsaw hospital between July 11th 2022 and April 21st 2023. The treatment group (TG) was hospitalized in the Department of Orthopedics and Rehabilitation due to fracture of the proximal femur. The control group (CG) was formed by the patients treated in the Department of Internal Medicine.
Study design
All the individuals were primarily included in the study if they met the following criteria: age over 65 years, fluency in Polish speaking, and in terms of the treatment group fracture of the proximal femur. The exclusion criteria were as follows: prevalent fracture, history of neoplastic disease, treatment of osteoporosis, mental illness and immune disease affecting protein balance. All the patients from the treatment group were found to be eligible for the surgery. According to the history all the fractures resulted from own-height falls which were actually low energy injuries. Those who suffered from femoral neck fracture underwent hemiarthroplasty (MultiPolar/Taperloc, ZimmerBiomet) or total hip arthroplasty (G7/Taperloc, ZimmerBiomet). Transtrochanteric fractures were fixed with the use of an intramedullary nail (Biomet Affixus). All the operations were performed by the same experienced orthopedic surgeon. In the control group in every case a blood sample from an elbow vein was drawn at the admission to the department, and in the treatment group immediately before being admitted to the operating theater. It was collected in EDTA-K2 tubes and then centrifuged (Eppendorf Centrifuge 5804 R; 1600 rpm) to separate plasma from the whole blood. The plasma was stored in the freezer at –4°C. Subsequently, the concentration of C-terminal cross-linked telopeptide of type I collagen (CTX-I; AC-02F1; Immunodiagnostic Systems Holdings Ltd.), SHBG (EIA-2996; Immunodiagnostic Systems Holdings Ltd.), CK (EIA-4112) was determined in plasma using commercial enzyme-linked immunosorbent assays (ELISA), according to the instructions provided by the manufacturer. All the variable measurements were conducted in the same laboratory (Center for Preclinical Research and Technology, Medical University of Warsaw, Poland). The obtained results were statistically analyzed.
Statistical analysis
Statistical analysis of obtained results was performed. All comparisons were performed between independent variables. To assess the correlation between given variables Spearman’s coefficient was used. For comparisons between continuous variables the Mann-Whitney U test with t-approximation was utilized. The α-value was set at 0.05. All statistical analyses were conducted using SAS software, Version 9.4 for Windows (SAS Institute Inc., NC, USA). For the results the mean and the standard deviation were reported for a normally distributed variable. Otherwise, the median with 25th and 75th percentiles (Q1 and Q3) was provided. The outliers were not excluded from the study. These values are depicted in the figures as hollow dots.
Bioethical standards
This study was registered at ClinicalTrials.gov with the registration number NCT05804604. Institutional Ethics Committee approval was obtained (approval number: KB/152/2021) and every participant signed written consent to participate. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Results
The TG consisted of 22 patients, and the CG consisted of 18 patients. Mean age of participants in the TG was 83.5 ±7.3 and in the CG 79.05 ±9.35 years. Mean body mass index (BMI) of patients in the TG was 22.6 ±1.8 and in the CG 23.4 ±1.4. Baseline characteristics of the participants are presented in Table I.
Table I
Baseline characteristics
| Parameter | Treatment group | Control group | p-value |
|---|---|---|---|
| Number of patients | 22 | 18 | – |
| Mean age [years] | 83.5 ±7.3 | 79.05 ±9.35 | 0.1 |
| Mean BMI | 22.6 ±1.8 | 23.4 ±1.4 | 0.37 |
| Female : Male | 15 : 7 | 11 : 7 | 0.64 |
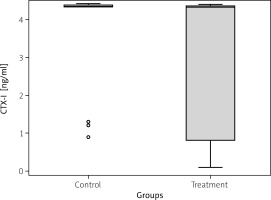
The analysis of the plasma samples revealed statistically significant differences between treatment and control groups in concentrations of all studied proteins. Plasma CK concentration varied between the studied patient groups: TG = 5.07 ng/ml (IQR = 3.7) vs. CG = 2.03 ng/ml (IQR = 2.5); p = 0.011 (Fig. 1). Additionally, the SHBG plasma concentration was significantly higher in the TG (5.7 nmol/l with IQR = 8.1) compared to the CG (2.3 nmol/l with IQR = 3.0); p = 0.006 (Fig. 2). In turn, a slight difference was found in plasma CTX-I concentration between groups: TG = 4.3 ng/ml with IQR = 3.6 vs. CG = 4.4 ng/ml with IQR = 0.1; p = 0.038 (Fig. 3).
Fig. 1
Creatine kinase (CK) plasma concentration in control and treatment groups. Median value is provided with the blue horizontal line within the box. Lower and upper green borders of the box correspond to Q1 (1st quartile) and Q3 (3rd quartile), respectively. Lower and upper whiskers reflect 1.5*IQR (interquartile range).
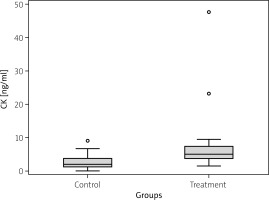
Fig. 2
Sex hormone binding globulin (SHBG) plasma concentration in control and treatment groups. Median value is provided with the blue horizontal line within the box. Lower and upper green borders of the box correspond to Q1 (1st quartile) and Q3 (3rd quartile), respectively. Lower and upper whiskers reflect 1.5*IQR (interquartile range).
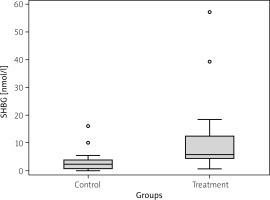
Fig. 3
C-terminal cross-linked telopeptide of type I collagen (CTX-I) plasma concentration in treatment and control groups. Median value is provided with the blue horizontal line within the box. Lower and upper green borders of the box correspond to Q1 (1st quartile) and Q3 (3rd quartile), respectively. Lower and upper whiskers reflect 1.5*IQR (interquartile range).
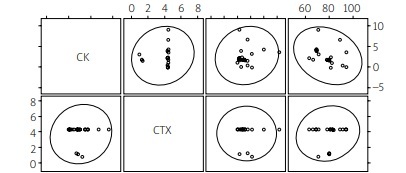
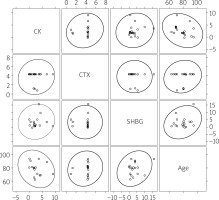
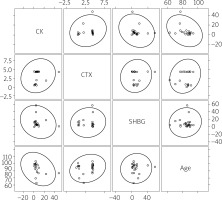
Additionally, no significant correlations were found between age, plasma concentration of CK and markers reflecting bone remodeling and conditions: SHBG and CTX-I in the TG as well as in the CG (Figs. 4, 5).
Discussion
The present study investigated any relationships between the breakdown of muscle tissue and the incidence of PFF. In addition, the bone quality of our patients was assessed. The most important finding of our research is that patients with this type of fracture tend to have a worse condition of the skeletal system than their counterparts.
In our study we conducted an analysis of three proteins. The first of these is SHBG, which is a glycoprotein playing an important role in regulating bone turnover and its mass [14]. It may indirectly affect fracture risk by binding estradiol and testosterone. As a result, the higher level of SHBG reduces the concentration of free and bioavailable sex glucocorticosteroids, lowering bone mass density [15, 16]. Patients with PFF had a significantly higher SHBG plasma level than those from the CG. That may indicate that the bone condition in the TG was basically worse. Similar observations were made by Varavsky et al. [17]; they proved that SHBG plasma concentration was related to bone mass and as a result vertebral fractures (VF). In their analysis as well as in ours, the higher the SHBG level was, the lower was the measured bone mass, which led to increased fracture prevalence. Their treatment group, however, consisted of patients with prostate cancer, which in our case was an exclusion criterion. Neoplastic disease, especially the type that metastasizes to the bones, affects the level of plasma proteins, including bone turnover markers [18, 19].
Nevertheless, their conclusions are consistent with a study from Vandenput et al. in which cancer patients represented approximately 10% of a large (more than 4,000) study group. They also concluded that a high SHBG level predicts the incidence of clinical and radiographic VF in elderly men, primarily. It also adds some information for VF risk prediction irrespectively of the other factors such as smoking, previous fractures, some chronic diseases, or alcohol abuse [15]. Our results demonstrate that the plasma concentration of SHBG is not influenced by age, which according to Cawthon et al. [20] could make it an independent predictive factor for fracture. Although the conclusions from our study are based on PFF rather than VF, the results are consistent with each other. What is interesting is the fact that in our study patients with PFF had higher levels of both SHBG and CK – a marker of muscle decay. Similar outcomes were obtained by Ellis et al. [21]. In their study it turned out older adult women with higher SHBG concentration had lower muscle mass and volume. Although we cannot draw the same conclusions, as in our study MRI examination was not conducted, nor was muscle index calculated, we are inclined to believe so.
C-terminal cross-linked telopeptide of type I collagen is considered a reference marker of bone resorption, which is a useful tool for fracture risk assessment and therapy monitoring in clinical settings [22, 23]. It reflects the activity of osteoclasts and bone remodeling [24]. Kirk et al. [25] reached the conclusion that higher CTX levels are related to poorer muscle function of the lower limb. Also in our study patients affected by fracture were revealed to have greater muscle decay, which was accompanied by higher level of CTX-I as well. With muscle damage their function is assumed to get poorer. That is a noteworthy finding, requiring a thorough analysis, especially considering age-related progressive sarcopenia, and the higher tendency to trauma associated with imbalance due to lowered muscle mass and strength [26]. Our results are consistent with a meta-analysis by Tian et al. [27] where an association between CTX-I and fracture risk was found. Also Greenblatt et al. [28] described in their study the role of bone turnover markers, including CTX-I, as a bone condition indicator, which may anticipate its fracture.
The literature shows several studies regarding the level of CK as a reference marker for muscle decay [29]. Its utility is prevalent in the laboratory tests during certain diagnostic processes [30, 31]. Any disturbance in muscle metabolism leads to a cellular cascade of events, starting with depletion of ATP, by ATPase pump dysfunction, ending with CK leakage into the circulation [32, 33]. An elevated plasma CK concentration would indicate previous tissue damage, and as a consequence, reduced volume of muscle tissue. In our research the level of CK in patients with PFF was nearly twice as high as in the CG. That would suggest the fracture itself is a direct cause of muscle injury. However, in 86% of the operated cases, muscles surrounding the hip and the area of the fracture seemed to be macroscopically intact as identified during visual inspection intraoperatively. Nevertheless, out of sight damage could not be ruled out.
Moreover, the fractures of the proximal femur were, as usual, not displaced themselves. They are also low-trauma injuries; hence no local tissue traumatization was observed. In addition, our patients were operated on within an average of 60 h (between 42 and 72 h), mostly because of a necessity of surgery delay due to permanent NOAC treatment. However, after such a time the level of CK should at least normalize [34, 35], so there was no need to assess its dynamics. In our study the differences in patients’ CK concentration between the extreme time points (42 h vs. 72 h) were slight anyway. Taking it into consideration, we can assume that the condition of muscle tissue in the TG was basically worse. Nevertheless, some studies suggested that sarcopenia-induced low quality of muscle tissue in elderly patients was manifested by lower levels of CK [36].
However, CK level itself may be affected by medications, race, physical activity or even temporary inflammation [37]. This applies especially to elderly patients, often burdened with multiple conditions. The group of medications most often associated with an increase in CK level is statins, but the increases described in the literature usually exceed the norm by at least 5 to 10 times, which we did not observe in our study. Additionally, no patient experienced an episode of rhabdomyolysis according to their histories. They had been taking statins or other drugs such as β-blockers or angiotensin II receptor blockers long before inclusion in this study, so they were under constant supervision by their GP, who did not detect any potential deviations in physical examination or laboratory tests.
In addition, researchers highlight a concomitant role of CXCL12α, CAF22 and osteonectin [38, 39]. Elevated plasma CXCL12α was previously associated with lower bone density and increasing age, which is often accompanied by sarcopenia [26, 40]. Osteonectin, on the other hand, emerged as a useful biomarker to predict sarcopenia indexes in chronic heart failure and chronic obstructive pulmonary disease affected patients. Therefore, CK is not a self-reliant predictive factor defining the quality of muscle tissue and requires additional evaluation of other, more specific biomarkers.
Study limitations
We are aware that our study has some limitations. First of all, we used experimental ELISA kits, not the ones that have clinical application. We found some discrepancies between the units of the analyzed protein levels compared to data from other researchers. Moreover, the number of patients enrolled could be a bit larger to make our results more representative.
Conclusions
Based on our study we can state that CK alone is not an appropriate marker for the clinical assessment of muscle tissue quality in patients with or at risk of PFF. We cannot unambiguously state whether there is any relation between the condition of muscles and the risk of fracture incidence. We can just observe the intensity of muscle decay in the group of elderly people. The quality of bone tissue in patients with PFF was clearly worse than in patients from the control group, which would certainly contribute to the fracture itself. The results of our study should be considered very preliminary, though. More extensive analysis including assessment of the patient for sarcopenia, reflecting real muscle quality and its relationship with lower limb fractures, is definitely needed to draw any further conclusions.