Introduction
Behçet’s disease (BD) is a chronic, multisystemic inflammatory vasculitis characterized by a relapsing-remitting course and diverse clinical manifestations. Although the exact etiology remains unclear, genetic studies have identified strong associations with human leukocyte antigen (HLA), particularly HLA-B51, as well as non-HLA genetic factors [1]. Behçet’s disease predominantly affects populations in the Middle East, Far East, and Mediterranean regions, although cases have been reported worldwide. The highest prevalence has been documented in Turkey, Iran, Saudi Arabia, Iraq, Israel, northern China, and Korea, leading to it being referred to historically as the “Silk Road disease” [2].
Behçet’s disease can involve nearly every organ system, with the mucocutaneous, ocular, vascular, articular, gastrointestinal, urogenital, pulmonary, and neurological systems most frequently affected [3]. Currently, there is no definitive laboratory test for BD; thus, diagnosis relies on established clinical criteria. Key diagnostic features include recurrent oral and genital aphthous ulcers, ocular inflammation, cutaneous lesions, vascular and neurological involvement, and a positive pathergy test [4]. Importantly, BD is diagnosed based on a comprehensive clinical assessment rather than a single manifestation, making it a diagnosis of exclusion.
The clinical presentation of BD is not only highly variable among individuals but also temporally heterogeneous, meaning the order and timing of symptom onset differ significantly among patients. Further complicating diagnosis, clinical characteristics may vary across ethnic and geographic populations. Given that BD diagnosis is reliant on clinical manifestations, understanding the chronology of symptom development is essential for improving disease recognition and management.
Therefore, the aim of this study was to assess the sequence of symptom onset and clinical findings in BD patients at a nationally recognized Behçet’s center and to explore the association between symptom progression and patient- or disease-related characteristics.
Material and methods
Patients who visited our clinic between March 2007 and March 2019 and were diagnosed with BD were included. Exclusion criteria were: patients with only a single clinic visit, patients who received an alternative diagnosis during their disease course, patients with < 2 years of follow-up, and patients with incomplete medical records.
Before 2006, BD diagnosis was based on the International Study Group Criteria (ISGC) [5]. After 2006, the International Criteria for Behçet’s Disease (ICBD) was used [4].
Demographic data at the time of BD diagnosis were recorded. All relevant clinic visits were reviewed to extract: initial symptoms and clinical findings leading to BD diagnosis, chronology of symptom onset and progression, and relevant laboratory data, including HLA-B5 and HLA-B51, when available.
Behçet’s disease diagnosis was confirmed by at least one experienced rheumatologist, while ocular lesions were diagnosed and documented by an ophthalmologist.
Statistical analysis
Statistical analyses were performed using SPSS Statistics for Windows, Version 26.0 (IBM Corp, Armonk, NY). A two-tailed p-value < 0.05 was considered statistically significant. Data were presented as follows: continuous variables as mean ±standard deviation (SD) or median and interquartile range (IQR, 25th–75th percentile); categorical variables as counts and percentages.
Comparisons between groups were performed using Student’s t-test for normally distributed continuous variables, the Mann-Whitney U test for non-normally distributed continuous variables, and the χ2 test for categorical variables. Pearson correlation analysis was used to assess the relationship between age and symptom development.
Bioethical standards
This study was approved by the Institutional Review Board (IRB) at Tehran University, which waived the requirement for informed consent (No. IR.TUMS.MEDICINE.REC.1397.162). The study was conducted in accordance with the principles of the Declaration of Helsinki. Following IRB approval, a retrospective review of patient charts was conducted at a nationally recognized BD referral center.
Results
A total of 2,615 patients were included in the final analysis. The mean age at BD diagnosis was 25.0 ±10.1 years (IQR: 18–32 years). Males comprised 58.7% of the cohort, with a male-to-female ratio of 1.4 : 1. The median follow-up duration was 26 years.
Clinical manifestations
Mucosal involvement was the most prevalent manifestation, affecting 99.1% of patients at any point during the disease course and presenting as the initial symptom in 92.9% of cases. Ocular lesions were the second most common manifestation, occurring in 58% of patients, though they were present at diagnosis in only 5.9% of cases. Skin manifestations were observed in 46% of patients during the disease course but were present at diagnosis in only 4.6% of cases (Table I).
Table I
Clinical manifestations and chronology of symptom development. Data are presented as number (percent) or mean ±SD. Findings that are considered diagnostic criteria according to ICBD are indicated in bold
The pathergy test was performed in 2,584 patients (98.8%), of whom 932 (36%) had a positive result. Human leukocyte antigen B5 testing was conducted in 2,556 patients, with 1,409 (55.1%) testing positive. Human leukocyte antigen B51 was detected in 1,238 (49.3%) out of 2,511 tested patients. Human leukocyte antigen B27 positivity was found in 179 (7%) out of 2,531 tested patients.
Regarding inflammatory markers, an erythrocyte sedimentation rate (ESR) of 20–40 mm/h was recorded in 842 patients (32.1%) at some point after diagnosis, while 251 patients (9.5%) had an ESR of 50–100 mm/h, and 16 patients (0.6%) had an ESR exceeding 100 mm/h. C-reactive protein levels were elevated in 364 (38.3%) out of 950 tested patients.
Symptom chronology and disease onset
In 2,337 patients (89.3%), BD initially presented with a single symptom, while 142 patients (9.3%) had 2 symptoms at onset, and 37 patients (1.4%) had 3 simultaneous symptoms. The most frequent symptom combination at onset was oral and genital ulcers, followed by oral ulcers and uveitis.
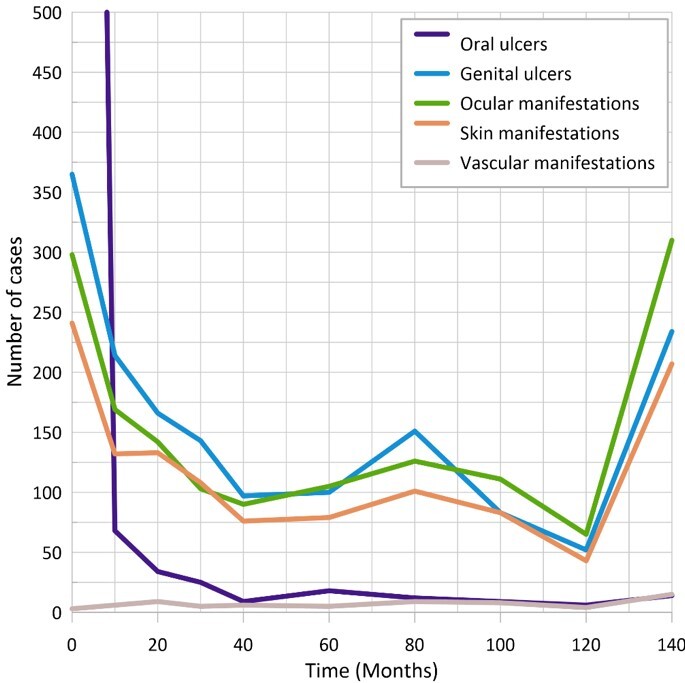
Table I summarizes the time from symptom development and BD diagnosis. Mucosal involvement was the most common initial symptom and had the shortest latency period. Oral ulcers were the only manifestation with a latency of less than 1 year.
Pairwise comparisons
Sex differences
The time from symptom development to diagnosis was significantly shorter in male patients for: oral ulcers (p = 0.04), ocular lesions (p = 0.04), deep vein thrombosis (p = 0.06), aneurysms (p = 0.03), and superficial phlebitis (p = 0.004). No significant differences were observed for other symptoms (Table II).
Table II
Effects of sex and age on time from diagnosis to development of manifestations of BD. Data are presented as mean years ±SD for sex. Significant comparisons are in bold
Age
Age was not significantly correlated with the time from diagnosis to oral ulcer development (p = 0.41). However, a negative correlation was observed between age and the onset of: genital ulcers (p < 0.001), with older patients developing them earlier in the disease course; skin manifestations (p < 0.001); and ocular lesions (p < 0.001). Additional details on age-related symptom progression are listed in Table II.
Human leukocyte antigen associations
Patients testing positive for HLA-B5 or HLA-B51 exhibited a significantly shorter time to the development of genital ulcers and skin manifestations. Other comparisons were not statistically significant (Table III).
Table III
Associations of HLA-B5 and HLA-B51 with symptom chronology. Data are presented as mean years from diagnosis to symptom onset ±SD. Significant comparisons are in bold
Initial symptom at diagnosis
Patients whose first manifestation of BD was oral ulcers had a significantly longer time to the development of additional symptoms across multiple ICBD criteria, as detailed in Table IV.
Table IV
Mean time from diagnosis to symptom onset compared between patients with and without oral ulcers as their first manifestation. Data are presented as mean years ±SD. Significant comparisons are in bold
Discussion
In this study, we aimed to evaluate the chronology of symptom development in patients with BD using one of the largest BD cohorts from a nationally recognized referral center. We assessed the prevalence of symptoms and clinical findings, the temporal sequence of symptom onset, and their associations with patient and disease characteristics.
Our findings confirm that oral ulcers are the most common BD manifestation, both overall (98.9%) and as the presenting symptom (90.3%) [3, 6, 7]. Genital ulcers were the second most common symptom (61.3%), though they were the initial presentation in only 8.9% of cases. Ocular lesions were observed in 58% of patients, but were the presenting symptom in just 5.9%. Skin manifestations, including pseudo-folliculitis (29.9%) and erythema nodosum (21.2%), were also frequently observed. Joint symptoms, though not included in BD diagnostic criteria, were present in 35.7% of patients. The distribution of initial symptoms in our cohort aligns with previous reports [2, 3, 7–15].
Unlike prior studies that primarily examined the time from first symptom onset to fulfillment of diagnostic criteria, our analysis extended beyond diagnosis to evaluate the long-term temporal development of symptoms. We observed substantial temporal heterogeneity among patients (Fig. 1, Table I), indicating that symptom onset occurs at varying intervals over time. Oral ulcers typically developed early, either as the first symptom or within the first year after the diagnosis, whereas other manifestations continued to emerge over 5 years or more. Notably, some symptoms developed over a decade after diagnosis, particularly ocular lesions (mean onset: 6.3 years) and uveitis (mean onset: 7.4 years). These findings underscore the necessity of prolonged patient follow-up to monitor for late-developing complications with significant morbidity, such as ocular and vascular manifestations [16–18].
We also identified sex-based differences in symptom development. Male patients exhibited a significantly shorter time from diagnosis to onset of oral ulcers (p = 0.04) and ocular lesions (p = 0.04). Additionally, vascular complications, including deep vein thrombosis (p = 0.06), aneurysms (p = 0.03), and superficial phlebitis (p = 0.004), developed more than 5 years earlier in males compared to females.
Age at disease onset also influenced symptom chronology. Older patients developed genital ulcers (p < 0.001), skin manifestations (p < 0.001), ocular lesions (p < 0.001), and vascular lesions (p < 0.001) earlier in the disease course. These findings highlight the potential impact of age at disease onset on clinical presentation and prognosis.
The association between HLA-B5/B51 positivity and BD manifestations has been well documented, with previous studies reporting a higher prevalence of genital ulcers, ocular lesions, and skin manifestations in HLA-B5/B51-positive individuals, particularly males [19].
Our results further support this, as HLA-B5/B51-positive patients developed genital ulcers (p = 0.004) and skin manifestations (p = 0.04) significantly earlier than HLA-negative patients, though other disease manifestations did not differ significantly between groups. We also found that patients who were positive for HLA-B5 or HLA-B51 developed genital ulcers and skin manifestations earlier in the disease course (p = 0.004 and p = 0.04, respectively), while other symptoms and disease manifestations were similar between groups.
Furthermore, we observed differences in symptom progression based on the initial presenting symptom. Patients who presented with oral ulcers as the first BD manifestation experienced a delayed onset of subsequent symptoms, particularly genital ulcers (p < 0.001), skin manifestations (p < 0.001), and ocular manifestations (p < 0.001). This delay is likely attributable to earlier diagnosis in patients with oral ulcers, as BD diagnosis often relies on the presence of multiple concurrent symptoms. Patients presenting with non-oral manifestations are more likely to receive a delayed diagnosis, as BD is often only considered once multiple criteria are met.
Study limitations
Our study has certain limitations. First, we did not assess the time from the onset of initial symptoms to BD diagnosis, as inconsistencies in symptom reporting and medical record documentation prevented reliable analysis. Additionally, some laboratory results, including HLA typing and ESR levels, were unavailable for all patients. Finally, we did not evaluate the effects of treatment strategies on symptom development, which could be an important area for future research.
Despite these limitations, our study has significant strengths. It includes a large, nationally representative cohort with long-term follow-up, allowing for a comprehensive analysis of BD symptom progression over time.
Conclusions
In this study of 2,615 BD patients, we identified significant temporal heterogeneity in the development of disease symptoms. While oral ulcers tend to appear early in the disease course, genital ulcers and skin manifestations often develop later. Ocular and vascular lesions exhibit the longest delay from diagnosis to onset, underscoring the need for long-term follow-up to monitor for late-developing, potentially severe complications.
Additionally, we found significant differences in symptom development based on sex, age, HLA type, and initial presenting symptom. Male patients exhibited earlier onset of multiple symptoms, while older patients experienced faster progression of genital, skin, ocular, and vascular manifestations. The HLA-B5/B51 positivity was associated with earlier onset of genital ulcers and skin manifestations. Patients presenting with oral ulcers as the initial BD manifestation had delayed development of other symptoms, likely due to earlier diagnosis.
These findings improve our understanding of BD’s natural history, emphasizing the importance of early recognition and long-term surveillance to optimize patient outcomes. Future research should further explore treatment effects on symptom progression and the genetic and immunological factors influencing BD manifestation timing.