Introduction
Treatment of severe rheumatoid arthritis (RA) is currently based either on biological agents or Janus kinase (JAK) and signal transducer of activators of transcription (STAT) inhibitors optimally used in combination with methotrexate (MTX).
The JAK-STAT pathway is the way of communication from transmembrane receptors to the nucleus. It serves to control gene expression and regulate cell growth and differentiation and uses diverse cytokines, interferons, growth factors, and regulated molecules.
Genetic polymorphisms and mutations are relevant to immune-related conditions and cancer. It has been confirmed by the development of a new class of therapeutics that target JAKs [1]. Janus kinase inhibitors (JAKi) modulate distinct cytokine pathways to different degrees and durations over 24 hours.
Several biological agents (biologics) have been approved for use in RA, revolutionizing the treatment. These biologics, which target cytokines such as tumor necrosis factor α (TNF-α), interleukin 6 (IL-6) or lymphocytes such as B or T cells, reduce functional disability and slow the progression of joint damage in RA.
Tumor necrosis factor inhibitors (TNF-i) are a group of drugs that suppress the body’s natural response to TNF-α. Tumor necrosis factor α is involved in the pathogenesis of RA and it is of major importance in RA [2]. Several clinical trials using TNF-α inhibitors have shown clinical benefit [3–11].
Among proinflammatory cytokines, IL-6, a pleiotropic cytokine, is also involved in the pathogenesis of RA. It is over-expressed in synovial tissue in RA patients, and affects the function of neutrophils, T and B cells, monocytes and osteoclasts – cells that are activated in RA. It is the inducer of the hepatic acute phase response. The anti-IL-6 receptor antibody tocilizumab (TOC) inhibits signaling of IL-6 [12–13].
Also B lymphocytes are of special importance in autoimmune processes and play a role in the activation of synovial T cells and induction of cytokine secretion. The success of B cell depletion therapy by using the monoclonal antibody rituximab (RTX), which targets CD20, highly specific to B cells, confirmed the importance of B cells in the pathogenesis of RA [14].
As T cells promote numerous disease pathways in RA, these cells are a target for anti-inflammatory therapy. Abatacept (CTLA-4Ig; ABA) is a soluble recombinant fusion protein which competes with CD28 for CD80 and CD86 binding and thereby can be used to selectively modulate T cell activation. Several clinical trials have confirmed the efficacy of this compound in the treatment of RA.
Although the development of disease-modifying antirheumatic drugs (DMARDs) and the introduction of TNF-α antagonists, TOC, RTX, and ABA have improved the clinical outcome of patients with RA, some patients have an inadequate response.
Among new treatment options of RA are JAK-STAT inhibitors, which are currently a modern, important and promising therapeutic option.
The aim of the study was to compare the effectiveness and side effects after treatment of biologic (b) DMARDs and targeted synthetic (ts) DMARDs.
Material and methods
Patients
One hundred and eight patients with active severe RA admitted to the Rheumatologic Outpatients Department of the Central Clinical Hospital of the Ministry of the Interior and Administration, Warsaw, Poland between January 2010 and September 2021 were finally incuded into analysis.
All patients met the RA American Rheumatism Association (ARA) criteria modified in 1987 [15] and 2010 EULAR RA classification criteria [16] (depending on the time of diagnosis) and with the radiographic confirmation of at least stage 3 RA according to Larsen and Dale’s scores for radiographic damage of the small joints (hands, wrist and feet) [17].
Patients with coexistence of other connective tissue disease or contraindications to intended treatment were excluded from this study. The research targets were patients with RA, resistant to MTX. Depending on the treatment used, two groups of patients were selected:
group I (n = 80 patients) treated with MTX 25 mg per week and biologic agents,
group II (n = 28 patients) treated with MTX 25 mg per week with JAK-STAT inhibitors.
In both groups, a concomitant stable dose of glucocorticosteroids (GCs) (10 mg of prednisone or less) was applied.
Clinical assessment
The duration of morning stiffness and the number of tender and swollen joints out of 28 (10 proximal interphalangeal joints, 10 metacarpophalangeal joints, 2 wrists, 2 elbows, 2 shoulders and 2 knees) were recorded. The Patients’ Global Assessment (PtGA) was assessed using a Visual Analogue Scale (VAS) (0–100 mm).
A routine blood test including basic inflammatory parameters such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) and urine tests were performed. From the obtained data 28-joint Disease Activity Score (DAS28) [18] and Simplified Disease Activity Score (SDAI) [19] were measured. Before the start of bDMARDS and tsDMARDs treatment, X-rays of the hands and feet were taken for radiologic assessment of the initial disease stage according to Larsen and Dale’s criteria.
The bDMARD and csDMARD drug history, including drug switch and combination therapy, was analyzed prior to study inclusion for each patient. According to remission or low disease activity (LDA) criteria all included patients in the study were assessed and the frequency of adverse events was compared between groups.
Statistical analysis
All results for categorical variables are expressed as counts and percentages, and Fisher’s exact test was used for comparison of proportions. Continuous variables are reported as mean ±SD (normally distributed data) or median and quartiles (Q1:25th–Q2:75th percentiles) in the case of skewed distribution.
The differences between continuous variables were tested by Student’s t-test or the nonparametric Mann- Whitney test, as appropriate. Fisher’s test was applied to examine the homogeneity of variance. Within-group comparison was done using the paired Student’s t-test or Wilcoxon test. All hypotheses were two-tailed with a 0.05 type I error. All statistical analyses were performed using SAS 9.4.
Ethical standards
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the local bioethics committee (consent No. 529/2012). The consent of Eli Lilly to include the baricitinib (BARI) group in the presented analysis was obtained; the results of the Eli Lilly clinical trial have already been published. All patients provided written informed consent before entering the study.
Results
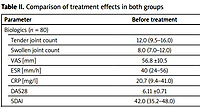
Table I presents demographic data and disease characteristics of studied patients. No statistically significant differences in disease duration, the duration of morning stiffness, the number of tender and swollen joints, ESR and CRP, VAS, DAS28 and SDAI results were noted between studied groups before the analyzed treatment.
Table I
Patients’ characteristics before and after treatment
[i] BIO – biologic therapy, CRP – C-reactive protein, DAS28 – Disease Activity Score of 28 joints, ESR – erythrocyte sedimentation rate, GCs – glucocorticosteroids, JAK-STAT inhibitors – Janus kinase and signal transducer of activators of transcription, MTX – methotrexate, SDAI – Simple Disease Activity Index, VAS – Visual Analogue Scale.
However, the patients treated with JAK-STAT inhibitors (group II) used a higher number of csDMARDs and bDMARDs than the patients from group I. Patients treated with tsDMARDs achieved lower values of VAS after treatment (p < 0.001) (Table I).
In group I in the medical history before starting biological agents (apart from MTX, which was used in all patients), 28 patients were treated with leflunomide (LEF), 56 with sulfasalazine (SF), 34 with antimalarials and 13 with gold salt.
In group I TNF-α inhibitors were used in the majority of patients, 93.75% (n = 75), as a first line biologic therapy. Among them 30 patients were treated with infliximab (INF), 24 etanercept (ETA), 14 adalimumab (ADA), 6 certolizumab pegol (CZP), and in only 1 case golimumab (GOL). Tocilizumab was the first biological drug used in 5 patients.
After the first choice of biologics stable remission was observed in 18 cases (in 7 cases after INF treatment, 8 after ADA, one each after CZP, ETA, GOL). In prolonged observation of the biological group that received anti-TNFs no remission or LDA was observed in 76% (n = 57) of patients.
The expected effects were not found after INF in 23 patients; therefore 6 patients were switched to ADA, 6 to ETA, 1 to CZP, 1 to GOL, 1 to TOC and 8 to RTX. Similarly, the target was not reached in patients treated with ETA (n = 23); therefore 12 patients were switched to ADA, 2 to GOL, 2 to TOC and 7 to RTX. There were no sufficient treatment effects after ADA in 6 patients; among them 3 patients were switched to RTX, 1 to CZP, 1 to GOL, and 1 to TOC. There was no sufficient effect after CZP in 5 patients and among then 3 were switched to TOC, one to GOL and 1 to RTX.
For the second choice of treatment TNF-i were used in 31 patients, ETA was used in 6 cases, ADA in 18, GOL in 5 and CZP in 2. Successful effects were obtained in 14 patients but 17 patients were switched to another biologic drug (11 to RTX and 6 to TOC).
The following side effects after TNF-i treatment were observed: after intravenous infusion of INF symptoms of allergy in 2 patients and lupus-like syndrome in 1 patient; after GOL treatment breast cancer was reported in 1 patient. There were no side effects after other analyzed TNF-i besides transient non-serious infections. There were no sufficient treatment effects after CZP in 5 patients, amoung them 3 were switched to TOC, 1 to GOL and 1 to RTX.
For the second choice after TNF-i, RTX was applied to 19 patients and TOC to 7 patients. As a third choice RTX was used in 11 patients. In total 30 patients were treated with RTX, among them 29 after failure of TNF-i and 1 patient after failure of TOC. No treatment effect after RTX was found in 12 patients. Side effects after RTX such as effusion allergy and syncope were observed in 2 cases.
As a first choice drug TOC was used in 5 patients, for the second choice TOC was used in 7 patients and for the third choice was used in 6 patients. In total 18 patients were treated with TOC, among them 7 failure of TNF-i and 6 patients after failure post RTX. The following side effects after TOC were observed: severe herpes zoster meningitis (1 case). The cancer was confirmed in three cases (uterine body carcinoma – 1 case, thyroid gland carcinoma – 1 and breast cancer in 1 case).
In group II after failure of MTX monotherapy JAK-STAT inhibitors were applied in 28 patients and among them 24 patients used BARI, 22 upadacitinib (UPA) and 8 used TOFA (4 of them after treatment with biologics and BARI). The group consisted of patients unresponsive to MTX monotherapy (n = 35) and to combined therapy with MTX and biologic agents (n = 15). In this group in the medical history before starting JAK-STAT inhibitors (apart from MTX, which was used in all patients), 17 patients were treated with LEF, 33 with SF, 7 with gold salt, 22 with antimalarial drugs. Finally 28 patients treated with JAKi were included into analysis (24 BARI, 4 TOFA and among them 4 with both BARI and TOFA). Patients treted with UPA were excluded from analysis because the summary of the clinical trial with this group of patients has not yet been published by sponsor (AbbVie).
As the therapy after failure of biologics, JAK-STAT inhibitors were introduced in 15 cases. As the second line therapy BARI were used in 4 patients in 1 after INF, 2 ETA and 1 after TOC. Two of them – 1 after ETA and 1 after TOC – did not achieve remission post BARI and were switched to RTX with good effect.
As the third line therapy JAK-STAT inhibitors were used in 8 patients with good results: 5 were treated with BARI and 3 with tofacitinib (TOFA). Before BARI the patients used INF, ETA 2 cases, INF, RTX in 1, INF, ADA in 1, ETA, RTX in 1, before TOFA – INF, GOL in 1 case, ETA, GOL in 1 case and ADA, TOC in 1 case.
As the fourth line therapy BARI was effectively used in 2 patients: after ETA, TOC, RTX in 1 patient, and after ETA, ADA, RTX in 1. As the fourth therapy TOFA was successfully applied in 1 patient after ADA, TOC and RTX.
Four patients who finished a clinical trial with BARI were switched to TOFA; however, in 2 cases the treatment had no effects and the patients were switched to ADA with a good response. In 1 case TOFA was withdrawn because of the serious side effect (intestine perforation).
In summary, finally 8 patients were treated with TOFA (4 after biologics and 4 after biologics and BARI), and 24 with BARI. No remission or LDA after treatment with JAK-STAT inhibitors was observed in 4 patients (2 after TOFA, 2 after BARI). Two patients who did not respond to BARI were treated with RTX and achieved LDA. Two patients after post-TOFA failure were treated with ADA with good effect.
In group II several side effects were observed as follows: 1 intestine perforation (TOFA), infection of left hip after alloplastic procedure 1 patient treated with BARI, transient leucopenia was observed in 1 patient with BARI and skin basal cell carcinoma in 1 patient with BARI.
Fatal side effects in 3 cases were also observed from group I, among them 1 after INF, 1 after sequential INF and RTX, and 1 after INF, ETA, RTX. In each of these events, cardiovascular complications were the cause of death.
The significant changes in all measured parameters in both groups before and after analyzed treatment are presented in Table II.
Table II
Comparison of treatment effects in both groups
Discussion
There is complete agreement among rheumatologists that the treatment for RA should be initiated as soon as possible in the early stage of the disease. The goal of the treatment is to achieve remission or LDA. Methotrexate is a drug of choice as a first line therapy and is widely used both in monotherapy and in combination therapy with other DMARDs as well as bDMARDs or tsDMARDs in the next stages of the treatment.
Experience from clinical trials with bDMARDs
Biological drugs are increasingly used, especially in patients with high disease activity. Among these drugs TNF-i are the best-studied group [3–11]. Other drugs with a different mode of action are ABA, TOC [12, 13] and RTX [14].
The superiority of the effectiveness of the combination of MTX with a biological agent over MTX alone has been confirmed in numerous randomized, double-blind, placebo-controlled phase III studies, e.g. ATTRACT [3], ASPIRE [4] BeSt COMET [5], TEMPO [6], ERA [7], ARMADA [8], PREMIER [9], RAPID1 [10], GO-BEFORE, GO-FORWARD [11] and GO-AFTER. The AMBITION [12] and ADACTA trials [13] demonstrated the superiority of TOC over MTX, and the DANCER and REFLEX studies [14] presented high efficacy of RTX.
The TEMPO (ETA) trial that included 682 patients [6], the ATTRACT (INF) study that included 428 [3], and the ARMADA (ADA) study with 271 patients [8] were randomized controlled trials with TNF-i conducted in patients with long-term RA. A number of studies have also been conducted in early RA such as the ERA (ETA) trial with 632 patients [7], the ASPIRE (INF) trial with 1049 patients [4] and the PREMIER (ADA) trial with 799 patients [9].
Randomization arms of research were as follows: the ASPIRE trial [4] used INF plus MTX in the following arms: 1 arm INF 3 mg/kg b.w. infusion weekly 0.2.6 and every 8 weeks plus MTX in 359 patients, 2nd arm INF 6 mg/kg b.w. infusions in the same weeks plus MTX in 363 patients and 3rd arm MTX up to 20 mg/week in 282 patients.
In the TEMPO study [6], ETA with MTX was also used in 3 arms: first arm ETA 25 mg twice a week SC plus MTX (231 patients), second arm ETA 25 mg SC twice a week in 223 patients, third arm MTX up to 20 mg/week in 228 patients.
In the PREMIER trial [9] ADA with MTX: first arm ADA 40 mg Q2W SC with MTX (n = 268), second arm ADA 40 mg Q2W SC (n = 274), third arm MTX up to 20 mg/week (n = 257 patients).
Mean age of patients in cited trials was 51, 53 and 52 years, disease duration (years) 0.9, 6.6, 0.8 and GCs use 38%, 61%, 36% of patients respectively. In all studies, the best results for the ACR 20, 50 and 70 criteria were achieved in the groups with combination treatment of MTX with TNF-i.
American College of Rheumatology (ACR) 20 criteria in the ASPIRE study [4] 3 mg/kg b.w. INF plus MTX were achieved in 62% of patients, INF 6 mg/kg b.w. with MTX in 66%, in the TEMPO study [6] ETA with MTX in 85%, and in the PREMIER study [9], ADA with MTX in 68%.
American College of Rheumatology (ACR) 50 criteria in the ASPIRE [4] study in the INF 3 mg/kg b.w. plus MTX group were achieved in 45% of patients, INF 6 mg plus MTX in 50%, in the TEMPO [6] ETA plus MTX study in 67%, in the PREMIER study [9] ADA plus MTX in 49%.
American College of Rheumatology (ACR) 70 criteria in the ASPIRE study [4] in the INF 3 mg/kg b.w. plus MTX group were achieved in 32% of patients, INF 6 mg/kg b.w. plus MTX in 37%, in the TEMPO study [6] etanercept plus MTX in 49%, and in the PREMIER study [9] ADA plus MTX in 47%.
Remission in early RA (DAS < 2.6) in the COMET trial [5] during 24 months assessing 542 patients was achieved in 50% of patients in the ETA plus MTX group vs. 28% in the MTX group. Seventy-one percent of patients in the ETA plus MTX group achieved ACR 50 vs. 49% of patients of the MTX group; 24% of patients on MTX and only 9% in the ETA plus MTX group stopped working during the study.
The GO-FORWARD study [11] evaluated the efficacy and safety of golimumab usage during 52 weeks in 444 patients with active RA despite MTX treatment. Patients were randomly assigned to receive placebo plus MTX (group 1), GOL 100 mg plus placebo (group 2), GOL 50 mg plus MTX (group 3) and GOL 100 mg plus MTX (group 4).
All injections were administered subcutaneously every 4 weeks. At week 52, 44%, 45%, 64% and 58% of patients in groups 1–4, respectively, achieved 20% ACR 20 criteria improvement; and 34%, 31%, 42% and 53%, respectively, achieved low disease activity (≤ 3.2) according to the DAS28.
The RAPID 1 trial [10] assessed the efficacy of CZP in 982 patients who were randomized to the following groups: placebo plus MTX in 199 patients, CZP 200 mg every two weeks plus MTX in 393 and CZP 400 mg plus MTX in 389. An initial dosage of 400 mg was given at weeks 0, 2, and 4, with a subsequent dosage of 200 mg or 400 mg given every 2 weeks, plus MTX, or placebo plus MTX.
At week 24, ACR 20 response rates for the CZP 200 mg and 400 mg groups were 58.8% and 60.8%, respectively, as compared with 13.6% for the placebo group. Differences in ACR 20 response rates vs. placebo were significant at week 1 and were sustained to week 52 (p < 0.001).
The side effects after TNFi treatment are infections (bacterial sepsis, opportunistic infections such as tuberculosis, atypical mycobacterium, fungal infections, Pneumocystis jirovecii, Listeria), demyelinating syndromes, hematologic toxicity, liver toxicity, lupus-like syndromes, cardiac heart failure worsening, skin cancer.
The AMBITION study [12] evaluated the efficacy and safety of TOC monotherapy vs. MTX in patients with active RA for whom previous treatment with MTX/biological agents had failed. Tocilizumab was better than MTX treatment with a higher ACR 20 response (69.9 vs. 52.5%, p < 0.001) and 28-joint Disease Activity Score (DAS28) < 2.6 rate (33.6 vs. 12.1%) at week 24 [20].
In the ADACTA trial [13] 163 RA patients were treated with TOC and 162 in the ADA group. In week 24 the mean change from baseline in DAS28 was significantly greater in the TOC group (–3.3) than in the ADA group (–1.8) patients (p < 0.0001). Tocilizumab monotherapy was superior to ADA monotherapy for reduction of signs and symptoms of RA in patients for whom MTX was deemed inappropriate.
The side effects after TOC treatment are infections, headache, elevations in liver aminotransferases and total bilirubin levels, elevations in total and LDL chole- sterol, rash, urticaria, hypertension, reduction in neutrophil count, cancer, GI perforation, hepatic failure, anaphylactic reaction.
The 24 week pivotal REFLEX study [14] examined 499 patients with active RA who had an inadequate response to at least 1 TNF inhibitor therapy and active disease (≥ 8 swollen and ≥ 8 tender joints). Patients were randomized to receive 2 × 1000 mg RTX infusions plus MTX or placebo plus MTX. American College of Rheumatology (ACR) responses at 6 months were as follows: ACR 20 criteria in RTX plus MTX group achieved in 51% vs. MTX group 18%, ACR 50 in RTX plus MTX – 27% vs. MTX 5% and ACR 70 in RTX plus MTX group 12% vs. 1% in MTX group.
The side effects after RTX treatment are fatal infusion reactions within 24 hours of its infusion, severe mucocutaneous reactions, some with fatal outcomes, hepatitis B virus reactivation, in some cases resulting in fulminant hepatitis, hepatic failure and death, progressive multifocal leukoencephalopathy resulting in death, tumor lysis syndrome, infections, cardiac arrhythmias and angina, bowel obstruction and perforation, cytopenia.
In summary of the results of cited trials, biologics are superior to csDMARDs but overall remission rates remain low. According to the RABBIT Registry (Germany) patients with RA newly treated with biologics or csDMARDs after failure of two or more csDMARDs achieved sustained remission only in 10% in 6 or 12 months. Also, overall sustained remission was achieved by less than 10% of patients receiving biologics, which is a relatively poor outcome [20].
Experience from clinical trials with tsDMARDs
The Janus kinase/signal transduction and activator of transcription inhibitors are also being introduced into treatment on a large scale, and their efficacy has been confirmed in many trials [21–38]. All JAKi studied so far in RA are effective: 3 have been approved [21–23].
The early onset of benefit with these drugs was observed, within 1–2 weeks, maximal at 3 months. Onset of action of TOFA, BARI and UPA is quite rapid, with an ARC 20 response as early as 1 week [21]. Significant reduction of PtGA, pain, Health Assessment Questionnaire (HAQ), duration of morning stiffness as early as 1 week [22], ARC 50 response as early as 2 weeks [23], PtGA and pain reduced as soon as 5 days [24].
Phase III clinical trials comparing JAKi to ADA in patients with RA with an inadequate response to MTX are as follows: treatment naive: TOFA – ORAL Start, BARI – RA-BEGIN, filgotinib (FILGO) – FINCH-3, UPA – SELECT-EARLY; MTX-IR: TOFA – ORAL Standard, ORAL Scan, ORAL Strategy, BARI – RA-BEAM, FILGO – FINCH-1, UPA – SELECT-COMPARE, SELECT-MONOTHERAPY; csDMARD-IR: BARI – RA-BUILD, UPA – SELECT-NEXT; bDMARD-IR: TOFA ORAL SelecOral Sync, ORAL Stop, BARI – RA-BEACON, FILGO – Finch 2, UPA – SELECT- BEYOND, SELECT-CHOICE.
In the ORAL Strategy trial 1152 adults with active RA despite MTX therapy were included in 1-year observation. Randomization arms were as follows: TOFA 5 mg BID (n = 384), TOFA 5 mg BID plus MTX (n = 376), ADA 40 mg Q2W plus MTX (n = 386). Primary endpoint ACR 50 response rate at month 6 showed non-inferiority of TOFA monotherapy and TOFA plus MTX vs. ADA plus MTX (independent comparison) [37].
In the ORAL STRATEGY trial [38] there were two arms; in one arm of 376 patients the effectiveness of TOFA was tested, in the second – 386 on ADA, Clinical Disease Activity Index (CDAI) ≤ 2.8 at month 6 was found in 14% of TOFA and 13% of ADA, and at month 12 in 19% TOFA and 17% ADA.
In RA-BEAM trial there were 1315 adults with active RA despite stable background MTX therapy. The study design was: BARI, ADA or placebo in patients with moderate-to-severe active RA and MTX-IR (an active comparator non-inferiority trial).
Randomization arms BARI 4 mg once daily (QD) (n = 487), ADA 40 mg once every 2 weeks (Q2W) (n = 330) and placebo (n = 488), after 24-week placebo were switched to BARI 4 mg QD. Adalimumab groups were switched to BARI at week 52. Primary endpoint BARI 4 mg QD plus MTX was superior to placebo plus MTX for ACR 20 at week 12. BARI 4 mg QD plus MTX was superior to ADA 40 mg Q2W plus MTX for change in DAS28-CRP and ACR 20 at week 12 [22].
The RE-BEAM study [22] assessed the effectiveness of BARI used in 487 patients compared to ADA in 330; CDAI ≤ 2.8 at month 3 was found in 8% and 7%, respectively, and at month 12 in 22%, BARI and 18% in ADA.
Another study, FINCH-1, included 1755 adults with moderate-to-severe active RA despite ongoing treatment with a stable dose of MTX – 4 arms: 1 – FILGO 200 mg QD plus MTX (n = 475), 2 – FILGO 100 mg QD plus MTX (n = 480), 3 – ADA 40 mg Q2W plus MTX (n = 325), 4 – placebo plus MTX (n = 475) in week 24 were switched to two groups: 1 – FILGO 200 mg plus MTX (n = 190), 2 – FILGO 100 mg plus MTX (n = 191).
Primary endpoint: FILGO 100 mg and 200 mg QD plus MTX were superior to placebo plus MTX for ACR 20 at week 12. Filgotinib 200 mg plus MTX was non-inferior to ADA plus MTX at week 12 for DAS28-CRP. Filgotinib 100 mg plus MTX did not achieve non-inferiority vs. ADA plus MTX for this measure.
The FINCH study [25] assessed the effectiveness of FILGO 100 mg used in 480 patients and FILGO 200 mg in 475 patients compared to ADA in 325; CDAI ≤ 2.8 at month 3 was found in 12%, 11% and 6%, respectively, and in month 12 in 30%, 24% and 23% respectively.
SELECT-COMPARE study design: 48-week randomized, double-blind treatment period (on stable background MTX therapy). Adults with moderate-to-severe RA and MTX-IR. Three randomization arms: 1 – UPA 15 mg QD plus MTX (n = 651), 2 – placebo plus MTX (n = 651), 3 – ADA 40 mg EQW plus MTX (n = 327). Primary endpoints (UPA vs. placebo) ACR 20 (FDA), DAS28-CRP < 2.6 (EMA) at week 12. After week 26 groups were on UPA 15 mg QD plus MTX or ADA 40 mg EQW plus MTX.
Primary endpoints: UPA 15 mg QD plus MTX was superior to placebo plus MTX for both primary endpoints at week 12, UPA 15 mg QD plus MTX was superior to ADA 40 mg Q2W plus MTX for ACR 50 and improvement in pain and HAQ-DI at week 12.
The SELECT-COMPARE study [26, 27] assessed the effectiveness of UPA used in 651 patients vs. ADA in 327; CDAI ≤ 2.8 at month 3 was found in 13.4% and 7.6%, respectively, and at month 18 in 28% UPA and 17% in ADA.
It is important to emphasize that the efficacy of JAKi vs. bDMARDs in MTX-IR patients was better: BARI 4 mg plus MTX was better than ADA plus MTX [22], UPA 15 mg plus MTX was better than ADA plus MTX [27] and TOFA 5 mg and FILGO 100 mg/ 200 mg plus MTX was non-inferior to ADA plus MTX [21].
In 5 studies [28–32] TOFA was administered at a dose of 5 mg twice daily (BID) or 10 mg BID, either as monotherapy or with background MTX treatment or another csDMARD. One of the studies included ADA 40 mg once every 2 weeks.
The assessment of the effectiveness of treatment was based on changes in parameters such as 4-variable DAS28 (with erythrocyte sedimentation rate (DAS28-4[ESR]), using the C-reactive protein level (DAS28-4[CRP]), CDAI, SDAI, and Boolean-based assessment.
A total of 3,306 patients were analyzed (1,213 of these patients received TOFA 5 mg BID, 1,212 received 10 mg BID, 679 received placebo, and 202 received ADA 40 mg every 2 weeks).
Remission rates varied according to the criteria used, with higher rates in the active-treatment groups for the DAS28-4(CRP) than for the other scores. At month 3 – re- mission rates with TOFA (5 mg twice daily) were 18–22% using the DAS28-4(CRP), 5–10% using the DAS28-4(ESR), 4–7% using the SDAI, 5–6% using the CDAI, and 2–7% using the Boolean-based method.
In contrast, the remission rates with placebo varied from 0% to 7%, with small differences between the DAS28-4(ESR) and the DAS28-4(CRP) [47]. At month 6 remission rates with TOFA were 25–40% using the DAS28(CRP), 7–15% using SDAI and CDAI, and 6–12% with the Boolean-based method.
In studies with BARI [22, 33] all 1305 patients were treated, among them: 488 with placebo, 487 with BARI and 330 with ADA. At week 12, the primary ACR 20 response rate for BARI (2 mg and 4 mg) was 70% as compared with 40% for placebo (p < 0.001).
At month 3 remission rates for BARI (2 mg and 4 mg/day) were 19–26% respectively, using SDAI 8–9%, using CDAI 8–10%, and 7% using the Boolean-based method. At month 6 remission rates using DAS28(CRP) were 11–15%, using SDAI 15–17%, using CDAI 15–16%, and using the Boolean-based method 11–12%.
The studies with UPA in a dose of 15 mg/daily achieved at week 12 the remission rate using DAS28 of 31%, using SDAI 12–14%, using CDAI 13%, using the Boolean-based method 7–10%. At month 6 the remission rate using DAS28(CRP) was 39–41%, using SDAI 16–20%, using CDAI 11–21%, and using the Boolean-based method 9–19% [34].
The studies with FILGO in the doses 100 mg and 200 mg/daily achieved at week 12 the remission rate: DAS 28–24%, SDAI 9–13%, using CDAI 11–12%, using the Boolean-based method 7–10%. At month 6 the remission rate using DAS28(CRP) was 15–48%, using SDAI 18–21%, using CDAI 19–21%, and using the Boolean-based method 14–19% [25, 35].
Inhibitors of JAK kinases (JAKi) were characterized by the shortest T½ among all DMARDs including biologics. A greater risk of reactivation or infection with herpes zoster virus (HZV) than with anti-TNF should be noted in the JAKi group. Despite HDL and LDL increase, the lipid profile is less atherogenic [35].
During JAKi therapy deep venous thromboses (DVTs)/venous thromboembolic events (VTEs) were observed. Incidence of DVTs/VTEs increased twice in RA with the range: 0.3–0.8/100 patient-years vs. DMARDs; bDMARDs [36].
These data derived from a Swedish registry and reported increases of these events depending on higher disease activity [37]. The majority of patients with VTEs had already had a prior event in the medical history [38]. Upadacitinib 15 mg and 30 mg: increased in phase 2 randomized controlled trials (RCTs), yet balanced incidence vs. ADA and MTX in phase 3 [26]. Tofacitinib 5 mg and 10 mg: 0.1/100 patient-years in RCTs and longitudinal observational studies [39]. In patients with baseline cardiovascular risk factors in comparison of reported incidence rates (IRs) of DVT and pulmonary embolism (PE) and the ratio of IR DVT/IR PE for TOFA (10 mg) were respectively 0.17, 0.24 and 0.71) [40].
A retrospective cohort study using 2010–2015 Market Scan Data, with ADA users (n = 6022), and TOFA (n = 2155), revealed VTE-ADA IR 0.83, TOFA 20 IR 0.83; the hazard ratio of VTE for TOFA was 1.07 (0.54–2.14) [41]. It was therefore concluded that in both the methods of treatment with JAKi and ADA as anti-TNF the risk of VTE was comparable between these groups.
Gastrointestinal perforations are more common than in TNF-i but less common than in TOC. Laboratory changes that require monitoring were leukopenia during TOFA, UPA treatment and neutropenia during treatment with BARI, FILGO and UPA [22, 26, 31, 33, 42].
Hemoglobin also decreases during UPA and BARI treatment but it was typically not clinically relevant [22, 26, 33]. During monotherapy with FILGO and UPA elevation of liver function tests (LFT) was also observed [26, 25]. Creatinine kinase increase reflects reversal of inflammation-induced inhibition of myoblast differentiation [22, 25, 26, 33, 42].
Serious infections with JAKi vs. bDMARDs were presented by Pawar et al. [43]. In the multidatabase cohort study there were 8 exclusive groups of RA patients initiating TOFA or bDMARDs using Medicare and Optum and Market Scan.
The primary outcome was admission to hospital for serious infections; finally 130,718 patients were studied and HR for serious infection – TOFA vs. ETA 1.41, vs. ABA 1.2 vs. TOC 1.17, vs. ADA 1.06 and lower than INF 0.81. Tofacitinib was associated with a 2-fold higher risk of HZV infection.
Regarding Janus kinase inhibition and reproduction, based on animal studies UPA may cause embryo-fetal harm when administered to pregnant women. Female patients of reproductive potential should be advised to use effective contraception during treatment with UPA and for 4 weeks after the final dose [23, 29].
The conducted comparative study is research that follows the convention of real life clinical trials (RCTs). The effectiveness in the presented cohort of patients after first line therapy was 24%; therefore in all groups 76% were switched to other bDMARDs due to not achieving the goal or due to the side effects.
Firstly, 37.5% of patients switched to other TNF-i and later 35.8% switched to RTX and 23.2% to TOC. In 15 patients there was an opportunity to switch from biologics to JAKi. Only 4 patients had no remission. Two patients were switched from BARI to RTX and 2 patients from TOFA to ADA. These 4 patients achieved remission. Side effects were more frequently noted after TOC and JAKi than other drug treatment.
Fleischmann et al. [44] evaluated efficacy and safety of immediate switch from TOFA to ADA or vice versa, in RA patients with non-response or incomplete response to the initial therapy. Among 659 RA patients on UPA and 327 on ADA therapy 39% and 49% needed alternative therapies respectively.
In both groups (ADA to UPA and UPA to ADA) and in non-responders and incomplete responders, improvement in disease activity was observed at 3 and 6 months. Low disease activity (CDAI) was achieved in 36% and 47% of non-responders and 45% and 58% of incomplete responders who were switched to ADA and UPA, respectively, 6 months following the switch [44].
The class of JAKi is an exciting development for rheumatology. Based on phase 3 RCTs, responses were better in progressively earlier disease duration, less treatment experienced patients, well-established effectiveness in RA, convenience, and short half-life. Adverse events can often resolve over a short time-frame. However VTEs, ATEs, surveillance for serious infections and malignancies must be taken into consideration.
Remission should be the treatment target for every patient. Remission in RA can be defined using multiple criteria: DAS28-CRP (4 variables), ACR/EULAR 2011 BOOLEAN definition of remission [45], ACR/EULAR index-based definitions of remission. In DAS28-CRP remission (4 variables) there was observed a stronger influence of tender joint count (TJC) than swollen joint count (SJC) and a high contribution of acute-phase reactant levels due to transformation and weighting.
In ACR/EULAR 2011 BOOLEAN definitions of remission the more stringent endpoint as the heavier weighting of TJC and SJC underestimated patient-reported improvement [45]. In ACR/EULAR 2011 index-based definitions of remission numeric addition of individual measures on their original scale was without transformation or weighting. The clinical disease activity index uses the same formula as SDAI except that CRP is excluded.
Remission rates vary depending on the criteria used: in DAS28-CRP the remission value is < 2.6, in CDAI ≤ 2.8, in SDAI ≤ 3.3, in Boolean yes/no. Boolean requires CRP values ≤ 1 mg/dl and PtGA of ≤ 1 on a 0–10 scale. ACR/EULAR Boolean remission is not widely utilized and rarely achieved, as it is a highly stringent definition of remission.
At any time point, the patient must satisfy all of the following: TJC ≤ 1, SJC ≤ 1, PtGA ≤ 1 on a 0–10 scale and CRP ≤ 1 mg/dl. In clinical trials, 61–66% of patients who achieved SDAI or CDAI remission also attained Boolean remission [45]. Biologics are superior to csDMARD but overall remission rates remain low; less than 10% of patients receiving biologics achieved sustained remission [20].
Achieving remission is the main therapeutic target in RA. The European League Against Rheumatism guidance recommends that treatment should be aimed at reaching a target of sustained remission or LDA in every patient. The European League Against Rheumatism/ACR Boolean or index-based remission definitions (CDAI or SDAI) are preferred.
Overall, remission rates are often below 30% in patients with moderate or severe RA. There are significant unmet needs for effective treatments that lead to sustained clinical remission or LDA.
Conclusions
The treatment with JAKi and inhibition of the JAK-STAT pathway show the same and may even exceed the efficacy of treatment with bDMARDs.
The oral form of drugs, as a non-invasive one, makes it easier for the patient to maintain treatment.
A short half-life of JAKi is advantageous in the case of planning surgical procedures or complications of such treatment.
Long-term observations from real clinical trials broaden the knowledge about their effectiveness. However, according to the current knowledge about side effects reported in clinical trials, such treatment should be closely monitored.