Introduction
Rheumatoid arthritis is an autoimmune disease affecting small joints and is characterized by progressive deterioration of bone and cartilage. Women are up to three times more likely to be affected, and it is more common in individuals > 40 years [1].
The global prevalence rate ranges between 0.24% [2] and 1% [3]. Prevalence of RA in the Western world is estimated at 1–2%, with approximately 2.3 million cases in Europe. A recent study in Poland revealed the prevalence to be 0.9%, concordant with the upper levels reported in Europe [4].
However, variations in diagnostic criteria hamper ascertaining the true prevalence of RA [1, 5]. A recent comprehensive study on the burden of RA across Europe revealed patient costs by treatment strategy to be €3000–€5000 per patient, and indirect costs per patient in the United States are reported to be $1000–$33000 [5].
For patients living with RA, progressive disability results in significant years-lost-to-disease. Thus, it is important to improve quality of life through better evaluation and treatment [2, 6].
The main goal in RA treatment is to achieve remission or low disease activity (LDA). For the last 20 years, the recommended standard-of-care for first-line treatment of RA has been the conventional synthetic disease-modifying antirheumatic drug (csDMARD) methotrexate (MTX), usually in combination with a short-term glucocorticosteroids (GCs) [7].
However, MTX treatment is not always successful due to lack of tolerance and efficacy. Failure of this treatment requires stratification, and an alternative DMARD, combined with a short-term GCs, can be used.
Adding a biological (b) DMARD [e.g. a tumor necrosis factor α (TNF-α) inhibitor], targeted synthetic (ts) DMARD (e.g. Janus kinase inhibitor), or interleukin 6 receptor (IL-6R) inhibitor is recommended after failure of two csDMARDs [8].
The decision to pursue either depends on the clinicians’ judgement, and tolerability and safety issues. The first-line biologic agents used in Poland are anti-TNF agents (infliximab, etanercept, adalimumab, certolizumab pegol, and golimumab) and the IL-6R inhibitor TCZ.
Tocilizumab is a humanized anti-IL-6 receptor monoclonal antibody, approved for treatment of RA in patients in whom treatment with at least one csDMARD or anti-TNF agents has failed [9, 10].
According to its summary of product characteristics (SmPC) it is indicated as a treatment for RA in adults, in combination with MTX or as a monotherapy. The effectiveness of TCZ monotherapy has been confirmed by several clinical studies: phase IV ADACTA [11], phase III AMBITION [12], phase III SAMURAI [13], and phase III OPTION [14].
Moreover, TCZ has also been shown to be effective in combination with MTX [15–20]. A recent sub-analysis of the ACT-SURE study [21] demonstrated that the efficacy of TCZ was similar in monotherapy and in combination with DMARDs.
Despite the availability of newer drugs such as TCZ and the proven efficacy of advanced regimens against RA, it is unclear how these have translated into the real-world clinical management of RA in Poland.
The main goal of our study was to obtain a better understanding of the management of RA with TCZ as a first-line biologic therapy in routine clinical practice in Poland.
Material and methods
Study design
This was a multicenter, non-interventional, prospective, observational study of adults with RA (ClinicalTrials.gov, identifier: NCT02234960). The aim was to evaluate the real-life effectiveness and tolerability of intravenous (IV) TCZ, 8 mg/kg b.w., Q4W, administered over a 6-month period in a clinical scenario in Poland.
Eligible patients were ≥ 18 years of age, had a diagnosis of moderate-to-severe RA, were either non-responsive or intolerant to prior DMARD treatment, and those for whom the treating physician decided to commence TCZ as first-line biologic treatment in routine clinical practice in Poland (patients were qualified by the Coordinating Committee for the Ministry of Health (MH) drug program for RA treatment) and in accordance with the SmPC (IV administration).
This included patients who had received tocilizumab treatment within the 4 weeks preceding the enrolment visit. In addition, study participants received all relevant patient information and provided informed consent.
The primary study objective was to establish the effectiveness of TCZ in patients, calculated as the percentage of patients with remission (DAS28 < 2.6) or LDA (DAS28 < 3.2) (effectiveness variables) after 6 months of treatment with TCZ.
Secondary objectives were to ascertain levels of remission or LDA after 3 months of treatment, changes in DAS28 over time, a comparison of monotherapy vs. a combination therapy with MTX, discontinuation rates, and changes in inflammatory parameters.
Patients were divided into sub-groups:
those with and without systemic symptoms at baseline, and
those administered with TCZ monotherapy or in combination.
The combination group was further divided into:
The exploratory aim was to investigate how comorbidities affect therapy outcomes with TCZ. The Rheumatic Disease Comorbidity Index (RDCI) [22] was used to quantify the total burden of comorbidity among patients.
Safety objectives were to identify and report all adverse events (AEs), serious AEs (SAEs), AEs of special interest (AESI), infections, administration reactions, dose modifications, discontinuations, and changes in laboratory parameters.
Demographic and RA disease characteristics on initiating TCZ were recorded along with any concomitant medication for RA use. Data were collected at baseline, and at 3 and 6 months (M3 and M6, respectively), to include all systemic RA features, age, and medical and treatment history of patients.
The data collected were based on clinical records and the usual standard of care for RA with TCZ specified by the MH drug program in Poland. This study was conducted in accordance with the protocol, the guidelines for Good Pharmacoepidemiological Practices (GPP), and Polish laws and regulations.
Statistical analysis
All quantitative variables were reported by providing the number of observations, mean, standard deviation (SD), minimum, first quartile, median, third quartile, and maximum as statistical parameters. When the distribution was not normal, the variable was transformed into its natural logarithm.
In that case the arithmetic mean of the logarithmically transformed variable was back-transformed into the geometric mean of the original variable. All binary or categorical variables were summarized by providing numbers and percentages. The impact of comorbidities on treatment outcomes was analyzed using Pearson’s correlation coefficient.
Ethical standards
All tests performed were related to the routine clinical practice of patients with RA treated with tocilizumab; all patients gave their written informed consent for the procedures. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki (ClinicalTrials.gov, identifier: NCT02234960).
Results
Enrolment characteristics
Our study enrolled a total of 101 patients over an 18-month period across 13 sites in Poland, and the total length of the study was 24 months. Patient characteristics are presented in Table I.
Table I
Patient characteristics at baseline (n = 101)
There were more female (n = 82; 81.2%) than male (n = 19; 18.8%) participants. The mean (SD) age was 53.2 (13.8) years (median: 56 years; range: 20–86 years). The mean (SD) duration of RA in the cohort was 8.2 (8.2) years (median: 5.1 years; range: 1–49 years) and was characterized by RA structural damage (59.4%), rheumatoid factor (RF; 82.2%), bone erosion (53.5%), and joint space narrowing (40.6%) in patients. The most common comorbidities were lipid disorders and hypertension.
Baseline characteristics
At baseline, patients were classified based on presence (n = 83) or absence (n = 18) of systemic signs of RA (Table I). At study onset, patient treatment regimens consisted of TCZ either as monotherapy (n = 29; 28.7%) or in combination with other agents (n = 72; 71.3%).
The latter comprised the sub-groups of TCZ plus MTX (n = 57; 56.4%), TCZ plus other DMARDs (n = 5; 4.9%), and TCZ plus MTX plus other DMARDs (n = 10; 9.9%). Increased C-reactive protein (CRP) levels, fatigue, anemia, and rheumatoid nodules were present in 56.4%, 43.6%, 32.7%, and 10.9% of patients, respectively.
A total of 44.8% received monotherapy due to intolerance of MTX, 20.7% received monotherapy due to AEs associated with MTX, and 17.2% received monotherapy due to contraindication for MTX.
Remission rates and disease activity at end of study
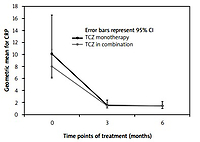
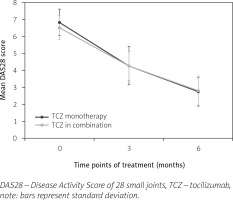
Remission rates at M3 and M6 were 6% and 31%, respectively, and comparable between TCZ either as monotherapy or in combination with MTX or any other DMARD. The low disease activity at M3 and M6 was 10% and 92%, respectively. Mean (SD) DAS28 decreased from 6.61 (0.74) at baseline to 4.27 (0.93) at M3 and 2.80 (0.86) at M6 (Fig. 1).
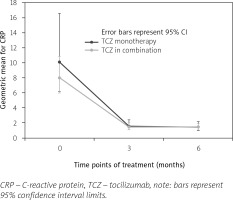
Similar decreases were reported for average CRP levels (baseline: 8.6 mg/l; M3 and M6: 1.5 mg/l) and erythrocyte sedimentation rate (ESR) (baseline: 24 mm/h; M3: 5.2 mm/h; M6: 5.1 mm/h). The proportion of patients presenting with systemic manifestations of RA decreased from 82.2% (n = 83) at baseline to 34.3% (n = 34) at M6.
Comorbidities and their impact on treatment outcomes
Forty-eight percent of patients had no comorbidities; 33% had one comorbidity, 14% had two comorbidities, and 5% had two or more comorbidities. The most common comorbid condition was lipid disorders, which were reported in 22% of patients, and primary hypertension, which was observed in 13% of the sample.
There was no correlation between baseline DAS28 and RDCI (p = 0.41). We found that RDCI does not affect the number of patients achieving LDA at M3 (p = 0.14) and M6 (p = 0.12).
When considering disease remission at M3, the correlation analysis revealed a positive relationship, at the borderline of statistical significance, between RDCI and a DAS28 of < 2.6 (p = 0.05).
A further prediction of a DAS28 value of < 2.6 based on the RDCI showed that there was 52% variance in achieving disease remission at M3. This can be explained by the variability of the RDCI (R² = 0.526; B = 1.69; p < 0.01).
The result indicates that the 52% degree of differentiation in terms of achieving full remission of the disease after three months is determined by the RDCI. The analysis revealed no correlation between RDCI and disease remission rates at M6 (p = 0.34).
Safety results
A total of 61 (60.4%) patients had 114 AEs, of which 86, reported in 41 patients, were judged to be mild (Table II). Five (4.4%) SAEs were reported in 3 patients. A total of 72 (63.2%) AEs were judged by the investigators as related to the study treatment. A total of 17 (14.9%) AEs in 10 patients were considered to be AESI.
Table II
Summary of adverse events
The most common AEs observed were lipid abnormalities and enhanced hepatic enzyme activity. At M6, abnormalities in high-density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TC), and triglycerides (TG) were reported in 29 (29%), 49 (50.5%), 61 (61%), and 24 (24%) patients, respectively.
In the case of HDL, values were either below the lower limit (n = 15; 15%) or above the higher limit (n = 14; 14%) of normal range. For low-density lipoprotein, TC, and TG, all abnormalities were as a result of values above the higher limit of normal range.
There was an increase in alanine aminotransferase (ALT) levels throughout the study. The mean increase from baseline was equal to 8.73 U/l and 5.60 U/l at M3 and M6, respectively, with a total of 26% of patients above the normal limit at M3 and 19% at M6. A total of 23% and 19% of patients had ALT levels > 1–3 times the upper limit of normal range at M3 and M6, respectively.
The post-baseline mean change in ALT levels revealed three patients at M3 had > 3–5 times the upper limit of normal (ULN) but no patients had > 5 times the ULN. For aspartate aminotransferase (AST) levels, there was an overall increase throughout study.
The mean increase from baseline was equal to 3.28 U/l and 3.12 U/l at M3 and M6, respectively, and 18% of patients were above the normal limit at M3, and 19% at M6.
All patients had > 1–3 times the ULN. For aspartate aminotransferase levels (mean post-baseline change), there were no patients with > 3 times the ULN at any timepoint.
Secondary effectiveness objectives
Secondary effectiveness objectives for the use of TCZ therapy in clinical practice in Poland across subgroups were defined by:
patient sub-types (systemic signs and symptoms of RA on entry to the study),
TCZ mono- vs. combination therapy with MTX, of which there were 3 further sub-divisions relating to the combination therapy.
In the first subgroup, systemic manifestations of RA tended to decrease from baseline to M6 (n = 83; 82.2% vs. n = 34; 34.3%), especially for fatigue (43.4% at baseline vs. 1% at M6). Changes in inflammatory parameters over time showed CRP levels above the ULN for 56.4% of patients at baseline vs. 5.1% at M6, and geometric mean ratios for CRP at M3 and M6 were numerically higher than in patients with no systematic signs of RA at entry (1.6 vs. 1.4 and 1.5 vs. 1.4, respectively) (Fig. 2).
The values of ESR at baseline were higher in patients receiving TCZ monotherapy (n = 29, 28.3) than combination therapy (n = 72, 22.4), but the geometric means were closer at M3 and M6 (4.9 vs. 5.3 and 5.3 vs. 5.1, respectively).
Changes in the treatment plan and concomitant medications
The treatment plan, regimen, and dose of TCZ were at the discretion of the patients’ physician. The post-baseline TCZ dose was modified for 6 patients (6%): 5 on combination therapy, 1 on monotherapy. The most common reasons for stopping previous treatment were due to AEs and intolerance in patients receiving concomitant MTX, and lack of efficacy in patients administered with other DMARDs where there was a reason for discontinuation of specific treatments.
Concomitant medication consisted of GCs therapy in 57.4% of patients, among whom the dose was reduced for 3 patients (3.0%) and withdrawn for 1 (0.9%) patient. A total of 66.3% of patients received MTX therapy, in which the dose was also reduced for 3 (3%) patients, and a total of 5 (16.1%) reduction events were reported. The reduction of MTX dose was due to anemia, hair loss, and prevention of renal function. In all MTX withdrawal cases (n = 3), AE were reported as the reason.
Discussion
This study assessed the effectiveness and tolerability of TCZ as first-line biologic treatment in patients with moderate-to-severe RA in routine clinical practice in Poland.
Assessing for the primary effectiveness variable of DAS28 after 6 months of treatment, we report a remission rate of 31% and LDA (DAS28 ≤ 3.2) in 92% of the patients. These were accompanied by substantial decreases in DAS28, CRP levels, and ESR. Most AEs reported were mild, and in general, TCZ seems to be a useful therapeutic option.
Several trials (AMBITION [12], OPTION [14], TOWARD [15], LITHE [16]) have demonstrated the efficacy of IV TCZ in first-line biologic treatment of RA. Moreover, the observed superiority of TCZ as a monotherapy in patients intolerant to traditional DMARDs has resulted in further studies. Two phase III trials (SUMMACTA and BREVACTA) led to the subsequent approval of subcutaneous (SC) TCZ as a non-inferior alternative to the IV route, providing an alternative administration route for patients to choose from [23–25].
The main objective of therapy in patients with RA is to achieve remission, or at least LDA, after 6 months [8]. When patients are intolerant to DMARD therapies, first-line biologics are the next step in treating RA. The European League Against Rheumatism (EULAR) guidelines [8] recommend the use of bDMARDs including TNF inhibitors (adalimumab, certolizumab, etanercept, golimumab and infliximab); abatacept (anti-CTL-4; a costimulatory inhibitor); TCZ (an IL-6 receptor blocker), and possibly in the future other IL-6 receptor inhibitors such as sarilumab and IL-6 inhibitors; and rituximab (anti-CD20, monoclonal antibody: an anti-B-cell agent).
These are recommended both as biological originator (bo) DMARDs and as European Medical Agency (EMA)-approved or Food and Drug Administration (FDA)-approved biosimilar DMARDs.
Worldwide, there are a limited number of patients on TCZ monotherapy, and recent results showed comparable efficacy in patients with RA for SC TCZ mono- and combination therapy with csDMARDs. The TOZURA multinational phase IV trial (n = 1804; 20% mono- vs. 80% combination therapy) reported how the low immunogenicity of SC TCZ supports TCZ monotherapy as an effective and safe treatment [26].
In 2009, less than 1.5% of patients with RA in Poland had access to biologic agents. Infliximab and etanercept were introduced in 1999 and 2000, respectively, adalimumab in 2003, rituximab in 2006–2009, and TCZ since 2009 [27].
In our study, the successful use of TCZ therapy in clinical practice in Poland was measured by the percentage of patients with remission (DAS28 < 2.6) and LDA (DAS28 ≤ 3.2) after 6 months of therapy.
Remission rates were 6% and 31% for M3 and M6, respectively, with no difference observed between mono- and combination therapy, including TCZ with MTX – an important point in line with randomized controlled trials and extension trials. The percentage of patients with LDA was 10% and 92% at M3 and M6, respectively, and this was higher at M3 and M6 for the monotherapy.
It was interesting to note that at M3, LDA was not achieved in patients (n = 15) receiving TCZ combination therapy which included DMARDs. Overall, a higher number of patients with LDA was observed in this study than previously reported in the SmPC (92% vs. approximately 50%), which may be a consequence of the smaller group size.
It is interesting how comorbidities affect treatment outcomes and the management of patients with RA. We found that RDCI did not correlate with the number of patients achieving LDA at M3 and M6 or with disease remission at M6. However, RDCI affected the remission rates at M3, which may mean that RDCI does not affect the results of 6-month treatment with TCZ.
In a study performed by Biggioggero et al. [28], where an anti-TNF agent was tested, elevated RDCI was a predictor of discontinuation of biologics and a lower RDCI score was a predictor of achieving a 12-month EULAR good-moderate response (p = 0.02), but not DAS28 LDA (p = 0.9) or remission (p = 0.09).
The presence of one comorbidity was not associated with 12-month LDA (p = 0.74) or disease remission (p = 0.48) [28]. As in our study, RDCI was not a predictor of achieving LDA or disease remission; however, measurements were performed after 12 months.
In contrast to these results, Batko et al. [29] reported that patients with higher RDCI had a lower chance of achieving LDA (OR 0.69; 95% CI: 0.61–0.79; p < 0.001).
The lack of influence of comorbidities on treatment outcomes in our study may be related to the high proportion of patients with no or one comorbidity.
In a study conducted by Burmester et al. [30], patients treated with adalimumab who had no or one comorbidity were more likely to achieve disease remission than those with two or more comorbidities.
In the earliest phases of RA, autoantibodies such as anti-citrullinated protein antibodies (ACPA) are known to be major risk factors for articular bone loss where ACPA positivity and high RF levels contribute to reduced systemic bone mineral density in patients [31].
In our study, RA characteristics at baseline were RF (82.2%), structural damage (59.4%), bone erosions (53.3%), joint space narrowing (40.6%), and other damage (5%) (Table I).
Previous therapies for patients included MTX (27.7%) and other DMARDs (86.1%), although patients had discontinued MTX due to AEs (6.9%) and intolerance (15.8%). For other DMARDs, discontinuation was mostly due to lack of efficacy (55.4%) (Table I).
Current therapy for all patients in this study was TCZ by IV (100%), either as a monotherapy or in combination with other agents.
Efficacy and safety data of TCZ are similar across studies in different patient populations, with reported remission rates of 59% at 52 weeks (SAMURAI trial) [13], 43% at 24 weeks (SATORI trial) [32], and 31% at 24 weeks in the study population, which is within the range of 30–40% in the SmPC.
Values were numerically similar for TCZ mono- (n = 28) and combination (n = 72) therapies (32.1% vs. 30.6%, respectively). However, a large difference was observed in reported LDA values at 24 weeks. In the RADIATE trial [17] LDA at 24 weeks was 51.2%, compared with 92% in the study population.
Values were also numerically higher for TCZ mono- than combination therapy (96.4% vs. 90.3%) and higher than in studies detailed in the SmPC (92% vs. approximately 50%).
In our study, the rate of infection was also lower than in other studies (5% vs. 11.1%), and this decreased rate of disease observed in the study population may be due to the small sample size [33]. The latter can also explain the low percentages of patients who experienced concomitant GCs or MTX dose reduction or therapy withdrawal that were observed in our study.
Our results confirm improvement in the effects of the RA therapy in Poland [34], especially in patients with comorbidities and who, for various reasons, cannot receive optimal treatment with MTX [35].
Study limitations
Limitations of this study are acknowledged where possible bias and confounding factors are introduced through the observational and non-randomized character of the study. However, there was no apparent source of bias in the estimated effectiveness of the study drug use in the selected patients’ population.
There was no control group, the study used only small sample sizes in subgroups, and potential sources of bias included limited monitoring, limited source data verification, and non-standardized laboratory analysis.
Another limitation is that, patients are qualified for biologic therapy based on the number of painful and swollen joints, as well as the Visual Analogue Scale include into Disease Activity Score, both of which are subjective in nature. Despite biological treatment, only a few physicians reduced the doses of CS and/or MTX, which can be considered a limitation of our data and analysis.
Another limitation of the analysis of comorbidities was the proportion of patients with no comorbidities (48%).
Conclusions
Our study confirms the effectiveness and safety of TCZ in real-world settings in Poland as a first-line biologic treatment, both as a monotherapy and in combination with other DMARDs in patients with moderate-to-severe RA who are either refractory or intolerant to DMARDs.
Importantly, comorbidities do not affect the results of 6-months treatment with TCZ, that is, the optimal time to achieve at least low disease activity, making it the drug of first choice in this group of patients.