Introduction
Systemic sclerosis (SSc), or scleroderma, is a rare autoimmune, connective tissue disorder associated with vasculopathy and fibrosis of the skin and internal organs [1–3]. Manifestations in the hands are common and may require additional treatment. Most SSc patients present with Raynaud’s phenomenon (RP) and digital ischemia and ulcerations.
Raynaud’s phenomenon is caused by vasospasm of the digital artery, which results in symptoms ranging from pain and paresthesia in mild forms to ulcers and gangrene in severe forms [4, 5].
The management of RP mostly relies on non-drug managements combined with pharmacological treatments, including calcium channel blockers, phosphodiesterase-5 (PDE-5) inhibitors, endothelin receptor antagonists, and prostacyclins [6, 7].
Surgical treatments such as peri-arterial sympathectomy and vessel repair are rarely used [8]. Another treatment option which has recently been suggested to have therapeutic effects on RP is botulinum toxin injection [6].
A few studies have examined the effect of botulinum toxin type A (BTX-A) in the treatment of RP. Most of these studies have included patients with primary and secondary RP [9]. There is some evidence suggesting that RP in SSc patients may be less responsive to BTX-A injections compared with patients with other underlying diseases [5, 10].
There are a few reports of studies that investigated RP only among SSc patients. Despite the conflicting data from one randomized clinical trial, other studies supported the effectiveness of BTX-A injection in the treatment of RP in SSc patients. However, there are still insufficient data on the efficacy of BTX-A injection for RP in SSc patients [11].
We conducted this parallel single-blinded, placebo-controlled clinical trial to investigate the therapeutic efficacy of local injections of botulinum toxin type A in the treatment of RP and its associated complications in SSc patients.
Material and methods
Study design and sample size
This study was designed as a parallel single-blinded, placebo-controlled clinical trial on 29 subjects at Imam Reza Hospital of Mashhad University of Medical Sciences (MUMS), Mashhad, Iran, between November 2018 and July 2019. Statistical data from the study of Bello et al. [10] were used to calculate the size of the investigated groups.
Participants
Participants were recruited among adults aged over 18, with SSc associated with bilateral RP (50 < score) based on the Raynaud’s condition score (RCS). The patients fulfilled the SSc ACR/EULAR classification criteria for SSc [12, 13]. Participants with an active infection in either hand, history of allergy to botulinum, upper extremity vascular surgery, splenectomy, pregnancy, and breastfeeding were excluded from the study.
Procedure
Written informed consent was obtained from each participant before enrollment. Participants underwent a complete physical examination and medical history. Each participant was injected with BTX-A (Dysport, Speywood Pharmaceuticals Ltd, Maidenhead, UK, 50 units in 2.5 ml sterile normal saline) in the non-dominant hand, referred to as the BTX-A group, and received sterile saline (2.5 ml) in the opposite hand, referred to as the placebo group.
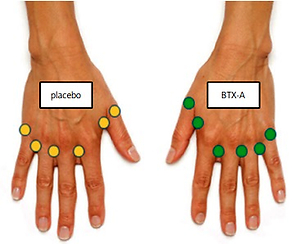
Injections were performed using a 1 ml syringe with a 31-gauge needle in 6 locations per hand; in the first, 2nd, 3rd, and 4th dorsal web spaces (10 units each), and the base of the thumb and the small finger (5 units each; Fig. 1).
Fig. 1
Injection sites: the first, 2nd, 3rd and 4th web spaces (10 units each), and the base of the thumb and the small finger (5 units each).
The participants were evaluated for digital pain, paresthesia, skin thickening, upper extremity function, Raynaud’s condition score (RCS), frequency of RP attacks, duration of RP attacks, ulcer number, and ulcer diameter.
All participants were blinded to the treatment allocation, and the injections were not visually distinguishable. The participants received their usual medications as previously prescribed.
Outcome measurements
Pre-injection measurements and post-injection follow-up at month 1 and 4 evaluations included patient-reported outcomes and physical examination performed by a rheumatologist.
Digital pain and paresthesia were evaluated by the VAS questionnaire [14], and modified Rodnan total skin score (mTRSS) [15] and Quick DASH questionnaire [16] were used to estimate skin thickening and upper extremity function.
We used the RCS questionnaire to evaluate the severity of RP, frequency of attacks, and duration of attacks.
Ethical standards
This trial has received ethical approval from the research ethics committee of the Iran National Institute for medical research development (protocol ID: IR.MUMS.MEDICAL.REC.1397.334) and also registered with www.IRCT.ir (registration ID: IRCT20081202001479N7).
Statistical analysis
We used the Kolmogorov-Smirnov (KS) test to evaluate the normality of quantitative data. Quantitative variables were described as means ±standard deviation (SD) and were compared using the paired Student’s t-test and Wilcoxon test. The ANOVA repeated measures test and the Friedman test were used to determine the variance of normal and abnormal variables.
All statistical analyses were performed using SPSS software version 16, and a p-value of less than 0.05 was considered statistically significant (Fig. 1).
Results
Among our 29 participants, the mean age was 41.21 ±12.52 years with female predominance (79.3%). Calcium channel blockers were the most used drugs (82.8%), followed by prednisolone (58.6%), mycophenolate mofetil (44.8%), PDE-5 inhibitors (41.4%), and a dual endothelin receptor antagonist (bosentan; 37.9%; Table I).
Table I
Demographic characteristics and drugs prescribed
The change of pain severity in a pre injection and on the follow-up in the 1 month after injection was significantly higher in the BTX-A group than the placebo group (p-value = 0.04). Between pre-injection and month 1 and month 4, the changes in the RCS (p-value = 0.02, 0.004, respectively) and the number of Raynaud’s attacks (p-value = 0.006, 0.001, respectively) were significantly greater in the BTX-A group than the placebo group.
No significant difference was reported in terms of paresthesia, skin thickening, upper extremity function, ulcer diameter, number of ulcers, or Raynaud’s attack duration between the two groups (p-value > 0.05; Table II).
Table II
Comparison of outcome changes from pre-injection to month 1 and 4 post-injection follow-ups in the hands injected with botulinum toxin A vs. placebo
In time, the decrease in pain severity, paresthesia, RCS, number of ulcers, and ulcer diameter, and the increase in upper extremity function were significantly greater in the BTX-A group compared to the placebo group (p < 0.05). No significant improvement was detected for skin thickening or the number of Raynaud’s attacks (Figs. 2A–2E).
Fig. 2
The comparison of pain intensity in hands injected with botulinum toxin type A vs. placebo in time (A). The comparison of RCS in hands with botulinum toxin type A vs. placebo in time (B). The comparison of paresthesia in hands injected with botulinum toxin type A vs. placebo in time (C). The comparison of upper extremity function in hands injected with botulinum toxin type A vs. placebo in time (D). The comparison of the number of ulcer in hands injected with botulinum toxin type A vs. placebo in time (E).

No adverse effect was observed in our study participants.
Discussion
Botulinum toxin type A is the most investigated botulinum toxin formulation and is suggested to have the highest potency. The evaluated dosage of BTX-A in previous studies ranged from 24 to 200 units per patient and a maximum dose of 100 units per hand. Botulinum toxin type A injection has been considered in three sites digital, distal palmar, and proximal hand [17].
In this parallel single-blinded, placebo-controlled clinical trial of 29 SSc patients with bilateral RP, we investigated the effect of BTX-A injection in dorsal web spaces on RP and its associated complications.
Our results demonstrated that BTX-A injection improved pain severity, paresthesia, upper extremity function, RCS, number of ulcers, and ulcer diameter in SSc patients. However, we did not detect any significant improvement in skin thickening or the frequency of Raynaud’s attacks. No adverse effect was observed among our study participants.
The first pilot study investigating the therapeutic effects of BTX-A injection on RP took place in 2004. It reported a beneficial clinical and laser Doppler interferometry outcome from BTX-A injections in two patients with primary and secondary RP [18].
Following this study, several reports described promising effects of BTX-A injection on clinical manifestations of RP and ulcers associated with RP. Beek et al. [19] in 2007 presented a case series of 11 patients and found BTX-A injection to be effective in the treatment of intractable digital ulcerations and rest pain in patients with severe vasospastic disorders, including two patients with SSc.
A retrospective study on 19 patients diagnosed with RP reported that 50 to 100 units of BTX-A injection improved tissue perfusion and reduced pain. In this study, three patients with SSc participated, of whom in two patients with diffuse disease, improvement in blood flow was seen but no symptom relief, and in one patient with limited disease, blood flow improvement and pain relief were reported [20].
A prospective study reported that BTX-A injection significantly improved RP symptomatology in patients with SSc despite the component of arterial sclerosis. In this study, improvement in ulcers, QuickDASH Score, O2 partial pressure, and pain were observed [21].
In another prospective study on 20 SSc patients treated with 100 units of botulinum toxin, 80% of the patients reported improvement in their symptoms, reduction in pain, and DASH score, 65% reported improvement in cold intolerance, 90% reported improved pinch grip, and 65% showed improved power grip [22].
A prospective case series of 10 patients with RP associated with SSc reported that 10 units of BTX-A injections decreased RCS and pain VAS. Skin temperature recovery after cold water stimulation at 4 weeks after injection was significantly enhanced. All digital ulcers in 5 patients were healed within 12 weeks after injection. Neither systemic nor local adverse effects were observed in all cases [23].
A systematic review in 2016 of 11 studies and a total of 125 patients reported that despite several promising studies, there is still insufficient data to evaluate the efficacy of BTX-A in RP [9].
The only performed randomized, double-blind, placebo-controlled clinical trial on 40 patients with SSc-associated RP showed no clear evidence for the beneficial effect of BTX-A in the treatment of RP. Similar to our study, patients received 50 units of BTX-A injections in one hand and sterile saline in the opposite hand. Clinical measures improved slightly for BTX-A hands in QuickDASH, McCabe score, pain VAS, and RCS. However, their laboratory-based LDI flow data did not support using BTX-A to treat RP in all SSc patients [10].
Currently, the exact underlying mechanism of the symptom relief and digital perfusion improvement followed by BTX-A injection is not fully known. Botulinum toxin type A has been shown to inhibit sympathetic activity, thereby inducing vasodilation. Botulinum toxin type A also inhibits acetylcholine and noradrenaline release.
Moreover, botulinum toxin reduces the expression of α-2C adrenoreceptors. Interestingly, in some patients, instant pain reduction occurs. This may be due to the inhibition of the release of pain-mediating neurotransmitters such substance P, glutamate, and calcitonin gene-related protein [19, 20, 24–27].
Study limitations
Our study had some limitations. Our results are mainly based on self-reported outcomes. A Doppler ultrasound evaluation for tissue perfusion could have improved the significance of our findings. Further randomization and blinding might have improved our results.
Moreover, the VAS and QuickDASH scales used in our study were not specifically designed for evaluating hand and upper extremity conditions in SSc patients. Finally, we did not assess the effects of the prescribed drugs in the evaluation of the therapeutic efficacy of BTX-A injection.
Conclusions
In conclusion, local injection of BTX-A showed to be safe and have a beneficial therapeutic effect on RP and RP-related digital ulcers in SSc patients.
Further, blinded randomized clinical trials with a larger sample size are warranted to confirm our findings in order to improve the life quality in SSc patients suffering from RP and its associated complications.