Epstein-Barr virus (EBV), belonging to the family Orthoherpesviridae (subfamily Gammaherpesvirinae, genus Lymphocryptovirus), is a ubiquitous human pathogen with a 170 kilobase double-stranded DNA genome closed within an icosahedral capsid surrounded by a protein layer called the tegument and enclosed by a lipid envelope containing glycoproteins with high expression of gp350 and gp220 [1].
The tegument helps the virus replication and evasion of the cell’s immune response. Epstein-Barr virus is well-adapted to humans, its only known host, and primarily targets B cells due to their expression of CD21, the principal entry receptor for the virus. However, it can also infect epithelial cells by utilizing the ephrin receptor A2. Following acute infection, EBV persists in a latent state, causing life-long infection.
Developing latency requires evasion of the immune response, which EBV achieves through blocking the expression of interferon genes, inhibiting complement activation, inactivating the cytotoxic functions of CD8+ lymphocytes, and inhibiting the host MHC class I antigen processing and presentation pathway [2]. During latency, the EBV genome usually persists in cell nuclei in the form of the multicopy, circular episome that associates with chromosomes during mitosis and is replicated by host DNA polymerase during the S phase.
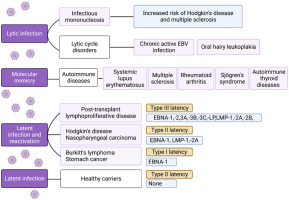
Different latent forms of EBV, varying in the transcriptional profile of non-coding RNAs and protein-coding mRNAs, have been identified (Fig. 1). Under conditions of altered cell-mediated immunity (induced by, e.g., stress, infections, immunosuppression), EBV reactivation can occur and not only lead to the onset of clinical symptoms and the virus becoming contagious but may also play a role in the pathogenesis of various diseases [3].
Fig. 1
The different clinical outcomes of EBV lytic and latent infection and EBV reactivation. Four types of latency can be distinguished (type 0, I, II, and III), differing in transcriptional profiles. Type 0 latency occurs in non-dividing memory B cells of healthy carriers, proteins are not expressed, and the expression is limited to two polyadenylated nuclear RNAs (EBER 1 and 2) and miRNAs. In latency type I, nuclear protein EBNA-1 is also expressed. Latent membrane proteins (LMP-1, LMP-2A) are additionally expressed in type II latency. In type latency III, all six EBNAs, three LMPs, two EBERs, and miRNAs are expressed. (Graph created with BioRender.com, accessed 08.08.2023).
Epstein-Barr virus, which spreads most commonly by saliva, infects 90–95% of the world’s human population, but such a high prevalence does not justify disregarding it as a significant pathogen. Firstly, EBV is a causative agent of infectious mononucleosis, most commonly affecting children, adolescents, and young adults [4]. Secondly, it is the leading cause of post-transplant lympho-proliferative disease [4].
Thirdly, it has been classified as a group 1 carcinogen, implicated in the etiology of Burkitt’s lymphoma, Hodgkin’s disease, nasopharyngeal carcinoma, some T cell lymphomas, and selected cancers of the stomach and smooth muscle (Fig. 1), and is responsible for 240,000–358,000 new cancer cases and 138,000–209,000 cancer-related deaths annually [5].
Epstein-Barr virus infection, particularly its reactivations, has also been implied in the autoimmune processes, acting as a trigger and/or a driver of selected autoimmune diseases (Table I). Further research is pivotal to elucidate genetic and environmental factors contributing to EBV-associated autoimmune effects [6].
Table I
Autoimmune diseases postulated to be associated with Epstein-Barr infection and reactivation
The links between EBV infection and autoimmunity are continuously being explored, as evidenced by the increasing number of peer-reviewed articles focusing on such associations. As of August 2023, 1,772 articles searchable in the PubMed database with the key words “Epstein-Barr virus” and “autoimmunity” were identified, among which 49% had been published since 2010.
In general, there are three main routes through which autoimmunity may be initiated in response to viral infection:
molecular mimicry route, which occurs when similarities between viral and host epitopes induce activation of autoreactive T or B cells by foreign-derived peptides,
bystander activation route, during which B and T (CD4+ and CD8+) cells are activated in an antigen-independent manner by signals that favor an inflammatory milieu, such as chemokines, cytokines, e.g., interleukin-extracellular vesicles with viral particles, ligands of co-signaling receptors, and pathogen-associated molecular patterns,
epitope spreading route, when a viral infection triggers the release of self-antigens, resulting in their uptake and presentation by antigen-presenting cells, eventually leading to de novo activation of autoreactive T cells targeting self-epitopes [11].
All three processes can occur in response to EBV infection and/or reactivation, ultimately leading to EBV-induced autoimmunity (Table I). The broad range and clinical relevance of the potential consequences of EBV infection fully justify the urgency to develop prophylactic vaccines and therapeutic strategies [12].
Epstein-Barr virus employs the gp350, gB, gH, gL, and gp42 envelope proteins to infect B cells, whereas BMFR2, gB, gH, and gL facilitate endothelial cell entry. Therefore, these envelope proteins may serve as antigen candidates in the design of vaccine triggering neutralizing humoral responses.
However, it is important that such a vaccine must simultaneously activate cellular responses with specific CD4+ (e.g., recognizing gp350) and CD8+ T cells (e.g., recognizing EBNA-2, EBNA-LP, and LMPs). Despite the discovery of EBV in 1964 and substantial efforts since 1976, there is no authorized vaccine against EBV. The first clinical trial of the prophylactic EBV vaccine, based on a recombinant vaccinia virus expressing gp350, was initiated in 1995 [13]. High hopes were associated with a candidate based on the recombinant envelope gp350 protein, which entered a phase 2 clinical trial.
Although it induced neutralizing antibodies in 70% of participants and reduced the incidence of infectious mononucleosis by 78%, it failed to effectively prevent EBV infection (NCT00430534). As of August 2023, only two prophylactic vaccine candidates have been actively tested in the clinical trials and registered in the ClinicalTrial.gov database. One is a vaccine based on gp350 ferritin nanoparticles adjuvanted with saponin-based Matrix-M1 (NCT04645147 and NCT05683834).
The second candidate in clinical testing (NCT05164094), mRNA-1189, was developed with mRNA technology and contains five mRNAs encoding envelope glycoproteins (gp350, gH, gL, gp42, and gp220) expressed in their native membrane-bound conformation. The aim is to produce a broad immune response that could reduce the rate of infectious mononucleosis and prevent EBV infection in different types of cells [14].
Specific therapeutic options targeting EBV are also currently unavailable. Antivirals’ failure to treat infectious mononucleosis can be attributed to the fact that disease symptoms do not result from viral replication but the immunological response to circulating and infiltrating EBV-infected B cells.
Preventing EBV reactivation from the latent to lytic phase to decrease associated sequelae, i.e., malignancies, autoimmunity, and post-transplant lymphoproliferative disorder, remains challenging. Directly acting antivirals or antivirals targeting cellular pathways that EBV interplays with, chemotherapeutic agents, and cell therapy based on EBV-specific cytotoxic T lymphocytes have been tested for such purposes with mixed results.
Several therapeutic vaccines, intended for use after primary EBV infection has already occurred, are currently in the pipeline. These include candidates based on transformed dendritic cells, viral vectors, and subunit vaccines that aim to induce immune responses against latent viral proteins [15]. A phase 1 clinical trial of the therapeutic vaccine candidate, mRNA-1195, was initiated recently (NCT05831111).
It contains mRNA molecules included in prophylactic mRNA-1189 candidate and additional mRNAs encoding latent antigens, although exact details have not been revealed at the time of writing. mRNA-1195 was initially intended to be tested in transplant patients to prevent post-transplant lymphoproliferative disorder, although it is likely that it will also be tested in patients suffering from autoimmune diseases, e.g., multiple sclerosis, in which chronic EBV infection is a driver or a trigger [14].
It is essential to ensure that a therapeutic vaccine does not include latent antigens which have increased potential for cross-reactivity, e.g., EBNA-1 (Table I).