Introduction
The treat-to-target (T2T) strategy is effective in reducing disease activity and joint damage in patients with rheumatoid arthritis (RA) and is currently a widely accepted guiding principle in treating RA [1, 2].
In treat-to-target, clinicians treat patients aggressively enough to achieve and maintain the defined goals of remission or at least minimal disease activity. However, success in treating inflammation has not always been accompanied by improved patient well-being, according to patient-reported outcomes (PROs) [3].
The reason could be the discordance between the needs of patients with RA and current therapy goals pursued by healthcare providers [4–7]. Therefore, we need to better understand patients’ unmet needs, preferences, and expectations, which are not adequately captured in current clinical practice [6–9]. Moreover, the current instruments for measuring disease activity and patient functioning endorsed by the American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) provide a limited mode of interpretation [10].
Identifying patient perceptions can improve the shared decision-making process, treatment adherence, and patient satisfaction with management decisions [2, 11]. It is of particular importance to empower patients who do not achieve treatment goals or experience disease symptoms such as pain and fatigue despite ongoing treatment. This plan covers a significant population because the RA remission rate under the T2T strategy is low in the real world [12].
To understand patient views, tools such as PROs and patient-provided information (PPI) questionnaires are implemented. Patient-reported outcomes denote the impact of disease and therapy on a patient’s life without any amendment or interpretation of the patient’s response [13, 14]. Patient-reported outcomes are incorporated into composite indices, such as the Disease Activity Score with 28-joint count (DAS28), Clinical Disease Activity Index, or Simplified Disease Activity Index in the form of the Patient’s Global Assessment of Disease Activity (PtGA).
However, PGA covering global health and overall disease activity does not reflect many aspects important from the patient’s perspective, such as different outcomes, endpoints, other attributes, and the trade-offs that patients are willing to consider [15]. Data collected using PROs and PPI are recognized as valid scientific evidence supporting the development and authorization of medicinal products [15–18].
Patient education is an essential determinant of the patient’s contribution to treatment because failure to understand the treatment strategy halts practical cooperation between the physicians and shared therapeutic decision-makers [19].
Education via the Patient Support Program (PSP) may improve the patient’s knowledge about the disease and treatment and positively impact patient adherence to self-care [20, 21]. The usefulness of online health resources depends on the patient’s digital health literacy (DHL), which comprises the ability to find, understand, and use information from online resources to make health-related decisions [22].
The study aimed to examine the satisfaction with treatment and the nature of therapeutic preferences and expectations of Polish patients with moderate to severe RA. We also collected DHL data to gain insight into patients’ ability to use health information from internet resources.
Material and methods
Study population
This report contains an analysis of Polish patients taking part in an international research (SENSE) study, who met the 1987-revised ACR or the 2010 ACR/EULAR classification criteria for RA. Patients were recruited from September 14, 2018, to May 31, 2019, in five Polish medical centers. All patients were treated with disease-modifying anti-rheumatic drugs (DMARDs) at study entry and had suboptimal disease control according to the disease activity indicator DAS28.
During one routine visit, patients were asked to complete PRO and PPI questionnaires. Clinical data were collected from patient medical records. Additionally, patient sociodemographic data, information about their comorbidities, current and planned treatment strategies, and planned modalities of treatment modifications were collected.
Measures
Patient treatment satisfaction was assessed using the Treatment Satisfaction Questionnaire for Medication (TSQM v 1.4) [23]. This instrument contains 12 questions and provides a validated score for four subscales: effectiveness, side effects, convenience, and global satisfaction. A predefined cutoff of ≥ 80 (out of a possible 100) on the global satisfaction subscale was used to determine treatment satisfaction.
A Visual Analogue Scale (VAS) was used to self-assess patient adherence to treatment, with a value ≥ 80 as an indicator of adherence [24]. Self-assessment of the severity of morning stiffness and pain in the last 7 days preceding the study was performed using VAS scores ranging from 0 (no stiffness or pain) to 10 (the worst possible stiffness or pain) [24].
The impact of RA on patient quality of life was assessed using the following validated PROs: the Health Assessment Questionnaire-Disability Index (HAQ-DI) [25], Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale [26], 36-item Short Form Health Survey v 2.0 [27], Physical and Mental Component Summaries (PCS, MCS), and the Work Productivity and Activity Impairment (WPAI)-RA v 2.0 [28] questionnaire.
The eHealth Literacy Scale was used to measure a patient’s DHL. The final score is the sum of all items ranging from 8 to 40, with higher scores reflecting a higher level of eHealth literacy. An eHealth score of < 26 indicates low DHL [24, 29].
Information on patient treatment preferences, expectations, and needs for a PSP was collected using questionnaires developed by AbbVie as previously described after obtaining consent to use the questionnaire [24].
Statistical analysis
Descriptive statistics were used to describe treatment satisfaction, expectations, and patient preferences using the full analysis set (FAS), which included all patients who fulfilled all inclusion criteria. Patient data and all PROs were assessed by subgroups, which were stratified by treatment, disease activity, and presence of comorbidities. Detailed statistical methods are described for the SENSE study [24].
Ethical standards
The study was conducted following the Declaration of Helsinki. All patients provided written consent to participate in the study and authorization to use their health data. The Bioethics Committee at the Silesian Medical Chamber, 49a Grażynskiego St., Katowice was informed. As the study is non-interventional, additional consent from the Ethics Committee were not required.
The authors obtained consent from the sponsor of the international trial (SENSE) to distinguish and use data of Polish RA patients included in the overall study.
Results
Patient characteristics
The SENSE study involved 1,624 patients with RA. This report contains an analysis of 52 Polish patients aged ≥ 18 years. Patient socio-demographic and clinical characteristics and the detailed RA medication (DMARDs) are presented in Table I.
Table I
Patient characteristics and medication for rheumatoid arthritis
[i] bDMARD – biologic DMARD, CDAI – Clinical Disease Activity Index, CRP – C-reactive protein, csDMARD – conventional synthetic DMARD, DAS28–CRP – Disease Activity Score in 28 joints with C-reactive protein, DAS28–ESR – Disease Activity Score in 28 joints with erythrocyte sedimentation rate, DMARD – disease-modifying anti-rheumatic drug, IQR – interquartile range, NA – not applicable, PhGA – Physician Global Assessment, PtGA – Patient Global Assessment, RA – rheumatoid arthritis, RF – rheumatoid factor, SD – standard deviation, SDAI – Simple Disease Activity Index, SJC28 – swollen joint count in 28 joints, TJC28 – tender joint count in 28 joints, VAS – Visual Analogue Scale.
Patient rheumatoid arthritis treatment satisfaction
Patient global satisfaction with the treatment subscale score indicates general dissatisfaction (Table II). However, 25% of patients scored above 82.1 on a global satisfaction subscale, which indicates their satisfaction with treatment. Detailed results of TSQM are presented in Table II.
Table II
Quality of life and work productivity indicators
| Patient-reported outcome | n | Mean (SD)a |
|---|---|---|
| Patient global satisfaction with the treatment, median (IQR) | 71.4 (50.0–82.1) | |
| Worst joint pain (0–10) | 52 | 5.6 (2.4) |
| Severity of morning stiffness (0–10) | 52 | 4.8 (2.4) |
| Duration of morning stiffness, hours, median (range) | 50 | 1.3 (0.1–6.5) |
| HAQ-DI score (0–3) | 52 | 1.0 (0.8) |
| FACIT-F score (0–52) | 52 | 28.1 (11.5) |
| SF-36 PCS (0–100) | 52 | 37.0 (6.4) |
| SF-36 MCS (0–100) | 52 | 43.8 (11.4) |
| WPAI-RA scores, %, median (range) | ||
| Presenteeismb | 16 | 30.0 (0.0–90.0) |
| Absenteeismb | 17 | 0.0 (0.0–100.0) |
| Total work productivity impairment | 16 | 37.8 (0.0–98.9) |
| Total activity impairment score | 52 | 45.0 (0.0–90.0) |
FACIT-F – Functional Assessment of Chronic Illness Therapy-Fatigue, HAQ-DI – Health Assessment Questionnaire-Disability Index, IQR – interquartile range, MCS – Mental Component Summary, PCS – Physical Component Summary, SD – standard deviation, SF-36 – 36-item Short Form Health Survey, WPAI-RA – Work Productivity and Activity Impairment-Rheumatoid Arthritis,
Impact of rheumatoid arthritis on patient quality of life
Rheumatoid arthritis negatively affects the physical health, mental well-being, and workability of patients (Table II). The median total work productivity impairment assessed in the employed patients (n = 16) was 37.8%. Additionally, the entire study group experienced a 45% impairment in total activity.
Patient digital literacy
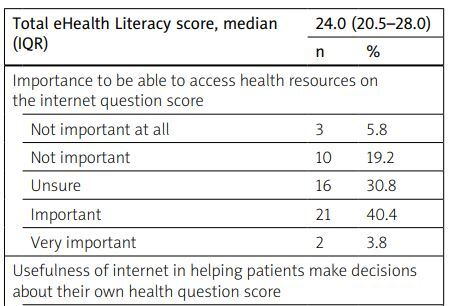
The median total eHealth literacy score in the study population was 24.0 (interquartile range [IQR]: 20.5–28.0, range 8–37), which implies low DHL (Table III). Patient ability to access health resources on the internet and views on the usefulness of the internet in making health decisions are documented in Table III.
Table III
Patient digital literacy
Patient treatment preferences
A significant percentage of patients preferred subcutaneous (46.2%) and oral (40.4%) over intravenous (13.5%) drug administration (Table IV).
Table IV
Patient treatment preferences
Regarding the frequency of dosing, most patients preferred monthly dosing for parenteral administration (57.7%) and once-daily dosing for oral administration (59.6%). The preferred time to the onset of drug effect was up to 1 week (48.1% of patients). Nearly half of the patients considered that treatment requiring a combination with another drug was acceptable once weekly. The most acceptable potential side effect was weight gain (42.3% of patients).
Patient need of support program
The most important aspect of a PSP for patients was access to educational materials about RA, RA treatment, and the PSP in general (Table V).
Table V
Patient need of Patient Support Program
The least important aspects for patients were the need to remind them about medical appointments and medication administration, support by a personal trainer, and digital lifestyle intervention.
Discussion
The results of our study indicate that moderately to highly active RA exerts a significant impact on patients’ lives. Study patients had high healthcare utilization reflected by frequent visits to professionals or hospitalization due to RA. They experienced significant work productivity impairment, and a high percentage of them (40.4%) retired early because of RA.
In addition, patient treatment satisfaction was low, which is consistent with the observations from numerous country-level and global studies which also show that patient treatment satisfaction is low, despite the widely accepted recommendations for implementing the T2T strategy, especially in poorly controlled RA [30, 31].
Patient treatment satisfaction has been found to be positively associated with biologic/targeted synthetic DMARD (b/tsDMARD) application and improved RA activity or physical function [31, 32].
In our study, only 40.4% of patients were treated with bDMARDs. Our patients did not receive tsDMARDs due to the lack of this treatment universal access within the study timeframe in Poland. It is worth mentioning that other factors not considered in this study, such as depression, could also have an impact on patient treatment satisfaction [33].
In line with the T2T approach, treatment plans should be adjusted if the treatment goals (i.e., remission or low disease activity) remain unachieved. Our study found that although patients had moderate or high disease activity, 51.9% of them were treated with DMARD monotherapy, mainly with conventional synthetic DMARDs (csDMARDs; 48.1%).
In addition, only 48.1% of patients were scheduled to switch to another DMARD (72% to bDMARD and 28% to csDMARD). These observations indicate a delay in making treatment modifications. A recent study found that sub-optimal treatment decisions may be common despite moderate to severe RA activity [34].
The lack of treatment intensification may be related to various factors, including the individual preferences of the patient or physician (e.g., comorbidities, clinical inertia), as well as the national healthcare organization and economic regulations [35, 36].
Our analyses have shown that patient treatment preferences were subcutaneous and oral over intravenous drug administration, monthly administration in the case of the parenteral route, or a daily single oral administration. The percentage of patients with RA who preferred oral administration of the drug was lower (40.4%) than those in other national (56.4%) [14], (64%) [36] or international (60.7%) [24] studies.
However, this result should be considered in the context of the lack of opportunity to benefit from the effects of oral Janus kinase inhibitors, which could have impacted the therapeutic preferences of study patients.
It was reported that concerns about adverse effects may influence patient treatment decisions [37]. Therefore, we asked our patients about the most and least acceptable side effects of RA therapy. We found that the most accepted side effect for patients was weight gain, and the least accepted side effects were increases in the risk of cardiovascular disease, infection, and malignancies. Our patient preference profile for RA treatment-related adverse events is consistent with previously published observations [37].
The patients wish to access educational materials on RA, RA treatment, and the patient support program, but without the need of a personal coach or digital lifestyle interventions – contradictory to the notion of digitization advancement in rheumatology [38].
A significant proportion of patients were unsure of the usefulness and importance of online health resources or were undecided in evaluating the aspects of their DHL (> 70% of patients showed low DHL), which is consistent with a previous report that showed that many patients with RA have insufficient skills to use internet health resources effectively [22].
The presence of elderly patients in our study might have influenced the level of digital literacy calculated for the entire cohort, as a negative correlation between DHL and the age of the respondents [39].
Study limitations
The limitations of our study should be acknowledged. Patient-reported outcome questionnaires may be prone to assessment bias. The patient-provided information evaluation did not assess the degree of patient preferences. Additionally, because we selected patients on the basis of their willingness to participate in the study, this study group may have had a more cooperative approach in contact with the physician compared to the general population of patients with RA.
However, although the study cohort was small, this investigation was part of an extensive international project (SENSE study), which involved 1,624 patients.
Conclusions
We provided data on preferences, expectations, and treatment satisfaction, along with broad socio-demographic and clinical characteristics of Polish patients with moderate or severe RA.
The results of this research were consistent with the study of a larger group of patients enrolled in the international SENSE trial, which included patients from different geographic conditions.
The results of this study should be of particular importance in clinical practice because they may influence treatment decisions and help adjust therapy in patients with sub-optimal control of RA. Consequently, it may improve the working life of patients and limit the utilization of healthcare resources. The study also provided unique DHL data of Polish patients with RA and advocated a necessary improvement.