Introduction
Coronavirus disease (COVID-19) is a new pandemic, which mostly affects the respiratory organs, although it produces an indirect effect on other organs and systems including the musculoskeletal system (MSS).
Musculoskeletal dysfunction after COVID-19 infection is found rather frequently in clinical forms of various degrees of general and muscular fatigue, myalgia, arthralgia, arthritis (about 50% cases) [1–3]. Nevertheless, its clinical-pathogenic features and therapeutic-preventive aspects are not sufficiently examined, especially those of arthritis.
Often, after achieving clinical improvement and verification of resolution of the viral attack by polymerase chain reaction (PCR) testing, patients have signs of damage to various organs and systems for a long time, which is defined as post-COVID syndrome (PCS) in the world literature lasting up to 6 months.
Long-COVID syndrome lasts longer [4, 5]. Clinical variability of PCS can involve any system, organs, and tissues [6–11]. Traditional methods of treatment of arthropathy are of low value in this situation, especially with arthritis. They are often limited due to their pathogenic features and restrictions because of comorbid diseases.
This highlights the necessity for further research to clarify the relationship between COVID-19 and development of inflammatory arthritis [1, 3]. The reumatological approach in rehabilitation of patients with PCS is in its initial period and requires further investigations [8, 10].
We aimed to investigate peculiarities of occurrence and description of musculoskeletal lesions in patients with PCS and improve the process of treatment of such patients.
Material and method
For the period from September 2020 to January 2022, we examined 142 patients with PCS in the form of stable manifestations of MSS lesions at the age of 36–67 years. During this period, the Wuhan strain dominated our territory, and from May 2021 – the SARS-CoV-2 delta strain. During the acute period, in all the patients examined PCR for SARS-CoV-2 was positive from a nasopharyngeal swab at the inpatient or outpatient stage of treatment.
Inclusion criteria
Criteria for inclusion in the study: absence of complaints and pain from the MSS before COVID, clinical manifestations of MSS lesions after experiencing an acute period of COVID-19, written consent to participate in the study. The scientific development considered the materials of extracts from the case histories of an inpatient during the acute period of COVID-19 infection.
According to these data, the degree of severity of the acute period was estimated as moderate, lung damage from 19 to 40% was detected in all the patients and did not require oxygen support. The therapeutic complex of the acute period was implemented according to the international recommendations.
It included detoxification means, glucocorticosteroids in average doses during 5–7 days, and non-steroidal anti-inflammatory drugs (NSAIDs). If bacterial lesions of the lungs appeared from day 4–5, inpatients received antibiotics. The average period of inpatient treatment was 12.1 ±1.2 days, and in the outpatient group (19 individuals) – 9.2 ±1.1 days.
In addition to carefully collected past history and standard clinical methods of examination of the joints and muscles, in order to specify peculiarities of MSS lesions we performed a series of tests: biochemical blood analysis with the levels of C-reactive protein, fibrinogen, antistreptolysin O (ASLO), rheumatoid factor, anti-nuclear bodies, antibodies to cyclic citrulline peptide, and HLA-B27 antigen.
To exclude reactive arthritis of another origin the following examinations were performed: antibody titers to Mycoplasma pneumoniae, Chlamydia pneumoniae, Chlamydia trachomatis, Ureaplasma urealiticum, Toxoplasma gondii, markers of hepatitis B and C viruses.
The severity of pain was assessed by means of the Visual Analogue Scale (VAS). The number of painful and swollen joints was evaluated. Morphological changes were assessed by means of radiographic and ultrasonographic examinations of the most afflicted joints. Clinical signs of PCS from other organs and systems (cardiovascular, respiratory, digestive, central nervous) were assessed in the point system: mild – 1 point, moderate – 2 points, severe – 3 points.
Considering an insufficient effect from the administration of non-steroidal anti-inflammatory drugs (NSAIDs) at the previous stage of treatment and other systemic clinical signs of PCS, in addition to the therapeutic complex 102 patients (the main group) received L-arginine (2,000 mg/day), L-carnitine (1,000 mg/day) and quercetin (240 mg/day) for three doses of medication. Other patients (40 individuals), who did not want to take them, were in the comparison group. Both groups of patients were representative in the age and gender aspects.
Treatment effectiveness criteria
Effectiveness criteria were rates of regression of joint and muscle lesions, time of incomplete (reduction of ache by half) remission, full recovery, the degree of tolerance to moderate physical exertion, and recurrence rate. The times of check-ups were 6 months later (52 patients), 12 months later (64 patients), and 18 months later (26 patients). Repeated face-to-face consulting was carried out 3–6 months later, between them every 3–4 weeks up-to-date telecommunication methods of monitoring were performed.
Statistical analysis
Statistical analysis was conducted using the program SPSS version 20.0. The normal distribution of the studied parameters was determined using Shapiro-Wilk’s test. The mean arithmetic value M and the average error m were determined. The difference between the studied values was determined by Student’s t-test. The statistical significance level was set at p < 0.05.
Results
Musculoskeletal lesions of the examined patients (arthralgia, arthritis) appeared 1–4 weeks after experiencing an acute period of COVID-19 infection of moderate severity. The reason for a family doctor to refer a patient to the rheumatologist was a limited effect of NSAID administration during 2–3 weeks after discharge from the hospital and occurrence of side effects after their use.
Age and sex features of patients with PCS and the signs of MSS lesions are presented in Table I.
Table I
Features of musculoskeletal lesions in age- and sex-stratified patients with post-COVID syndrome
| Features | Age group under 50 years (n = 47) | Age group older than 50 years (n = 95) | ||
|---|---|---|---|---|
| Males | Females | Males | Females | |
| Arthralgia [n (%)] | 21 (100%) | 26 (100%) | 43 (100%) | 52 (100%) |
| Arthritis [n (%)] | 10 (47.62%) | 11 (42.31%) | 31 (72.09%)* | 41 (78.85%)* |
| Visual Analogue Scale of pain [mm, total] | 42.2 ±1.92 | 39.2 ±1.68 | 54.8 ±3.12* | 53.1 ±2.84* |
| Number of painful joints in one patient | 2.0 ±0.0 | 2.0 ±0.0 | 4.0 ±0.0* | 6.0 ±0.0* |
| Number of swollen joints in one patient | 4.0 ±0.0 | 4.0 ±0.0 | 6.0 ±0.0* | 8.0 ±0.0* |
Table I shows that arthralgia was found in 100% of cases. The VAS score ranges from 39.2 ±1.68 cm to 54.4 ±3.12 cm with arthritis available. Its frequency in the age group older than 50 years was significantly higher (p < 0.05). Individuals under 50 years mostly complained of the knee joints (swelling, ache), but ache was determined with palpation of the ankle joints as well.
Patients older than 50 years complained of swelling and ache both in the knee, ankle and femoral joints (Table I). Regarding sex, occurrence of arthritis in females was non-significantly higher (p > 0.05). Clinical manifestation of arthritis was more pronounced in patients with considerable lesions of the lungs (30–40%) caused by SARS-CoV-2 in the acute period.
With respect to the muscular system, peculiarities of clinical signs in the examined patients were the following: quick muscular fatigue, decreased tolerance to moderate physical exertion, muscles remaining a long time in one position, ache in joints at night and stiffness in the morning lasting up to one hour.
We should note that in 27 (19.01%) patients aged 55–67 years, stage I and II involutional changes in the knee joins were radiologically detected (on the Kellgren and Lawrence scale), although they did not complain of joints ache before COVID-19. The BMI of these patients was within 22–27, and they were specialists of “sedentary” professions.
Peculiarities of involvement of joint structures into the pathological process with COVID-19 infection were the following:
mainly supporting joints reacted,
symmetry of lesions,
moderate signs of inflammation (swelling, local hyperemia, local temperature increase, synovitis signs),
in 2/3 cases arthralgia was associated with clinical signs of considerable muscle pain in the areas close to the afflicted joints,
more intense pain syndrome in the morning (starting character),
stiffness in the morning for 30–60 minutes,
the necessity to use larger doses of NSAIDs and for longer periods, more intensive protection of the stomach,
a decrease in the therapeutic effect of meloxicam group was noted among the examined patients, while nimesulide or paracetamol was better.
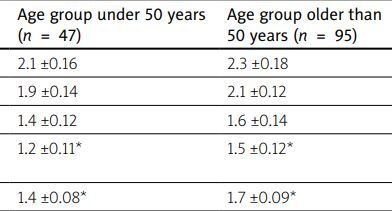
In all the cases MSS lesions were a part of a wide range of manifestations of PCS with dominating symptoms related to the cardiovascular, and digestive systems, respiratory organs and the central nervous system (Table II).
Table II
Comparative total features of post-COVID syndrome signs in the main systems and musculoskeletal system lesions (in points)
| Main system lesions and their manifestation | Age group under 50 years (n = 47) | Age group older than 50 years (n = 95) |
|---|---|---|
| Cardiovascular lesions (pain, palpitation, dyspnea, heart failure) | 2.1 ±0.16 | 2.3 ±0.18 |
| Digestive lesions (stomach ache, dyspepsia, enteropathy) | 1.9 ±0.14 | 2.1 ±0.12 |
| Respiratory lesions (cough, dyspnea) | 1.4 ±0.12 | 1.6 ±0.14 |
| Central nervous system lesions (disturbances of sleep and memory, asthenic-depressive signs) | 1.2 ±0.11* | 1.5 ±0.12* |
| Musculoskeletal lesions | 1.4 ±0.08* | 1.7 ±0.09* |
According to laboratory data in the blood of patients examined 4–6 weeks after discharge from the hospital, a 3–5-fold increase of C-reactive protein levels, 1.5–2-fold erythrocyte sedimentation rate (ESR) increase, moderate leukocytosis (9–15 × 109/l) with a moderate shift of the leukocyte formula to the left (8–18%), lymphopenia (p < 0.05) (Table III).
Table III
Markers of inflammation in the blood of patients with post-COVID syndrome and musculoskeletal lesions
| Examined parameter | Age group under 50 years (n = 47) | Age group older than 50 years (n = 95) | Laboratory control |
|---|---|---|---|
| C-reactive protein [mg/l] | 9.1 ±0.68* | 12.2 ±0.94* | 4.9 ±0.33 |
| Fibrinogen [g/l] | 4.88 ±0.32 | 5.21 ±0.26* | 3.5 ±0.18 |
| Leukocytes × 109/l | 12.38 ±1.12 | 14.55 ±1.14 | 7.2 ±0.48 |
| Rod nuclear neutrophils | 15.1 ±1.12* | 11.2 ±0.86 | 3.8 ±0.26 |
| Lymphocytes | 21.2 ±0.82* | 19.4 ±0.62 | 29.4 ±1.52 |
| Erythrocyte sedimentation rate [mm/h] (by Westergren method) | 34.16 ±2.66 | 38.22 ±2.44* | 21.6 ±2.18 |
Administration of L-arginine, L-carnitine and quercetin in the therapeutic complex during 10–12 weeks promoted better tolerance and action of NSAIDs, quicker regression of pain syndrome signs and other post-COVID signs, which was reliably better concerning the group of comparison (Table IV).
Table IV
Results of comprehensive treatment of musculoskeletal system lesions in patients with post-COVID syndrome with and without L-arginine, L-carnitine and quercetin (period of observation up to 6 months)
| Effectiveness criteria | Main group | Comparison group (n = 40) | |
|---|---|---|---|
| Age group under 50 years (n = 37) | Age group older than 50 years (n = 65) | ||
| Time to reduce joints ache by half (day, %) | 10.2 ±1.12* | 13.4 ±1.14# | 19.2 ±1.43* |
| Time of complete disappearance of joint at rest (day, %) | 29.4 ±2.56* | 43.5 ±3.28# | 58.2 ±4.66* |
| Time of using NSAIDs (days) continuously | 12.3 ±1.26* | 17.6 ±1.43# | 28.4 ±2.12* |
| Time of using NSAIDs (days) “as needed” | 28.6 ±2.44* | 34.4 ±2.78# | 52.6 ±3.24* |
| Time to renew tolerance to moderate physical exertion (days) | 34.5 ±2.16* | 42.2 ±3.26# | 74.2 ±5.23* |
| Frequency of relapses, (cases, %) | – | 2 (3.08%)# | 4 (10.0%) |
However, even after three months of such treatment, patients reported a certain decrease in tolerance to them, occurrence of shortness of breath, and less often, dry cough. Patients with obesity, ischemic heart disease, and heart failure reported similar symptoms. This complex of treatment was extended up to 6 months, and remission was achieved.
After 6 months of observation, all the patients with arthralgia and myalgia, and 65 patients with signs of arthritis without age-related X-ray changes in joints had no complaints and no objective signs of joint damage.
However, after 8–12 months, 19 out of 27 patients with X-ray signs of osteoarthritis (OA) of stage I–II, initially due to the provoking effect of prolonged physical exertion, had an exacerbation of the type of classical OA. A follow-up X-ray examination of the knee join revealed osteophytosis, narrowing of the joint space and subchondral osteosclerosis.
Clinical and radiological dynamic observation suggested that the signs of arthritis in the post-COVID period in people older than 50–60 years later served as a trigger for clinical manifestation of OA of the affected joints and to change the therapeutic tactics. Thus, symptomatic slow-acting drugs for osteoarthritis (SISADOA) were added according to the recommendations accepted by the leading world rheumatology associations.
Discussion
We examined peculiarities of MSS lesions in a group of patients with PCS, who experienced an acute period of COVID-19 infection of a moderate severity, non-oxygen-dependent variant during the period of dominance of Wuhan and later delta strains of COVID-19.
Musculoskeletal system lesions were found to be a considerable part of PCS with dominating symptoms from the cardiovascular, and digestive systems, general fatigue, and less dominating signs from the central nervous and respiratory systems. All the detected signs had a greater severity with increasing age of the patients, which caused complications of the rehabilitation process concerning the treatment of MSS lesions in particular.
In the therapeutic complex for patients with arthritis caused by COVID-19 infection and significant manifestations, a combination of NSAIDs and glucocorticosteroids is considered effective [12]. In our study difficulties in the treatment of MSS lesions were caused by marked gastroduodenopathy including erosions, enteropathy aggravated due to an intensive pharmacological load in the acute period of COVID-19 infection, and antibiotics in particular, with possible occurrence of intestinal dysbiosis [9], and arterial hypertension [3].
Analyzing the achievements of scientists in the study of pathogenesis of PCS [12–15] we noted that at the basis of the detected phenomena of systemic deterioration of manifestations, in particular, a decrease in the resistance of the mucous membrane of the digestive organs, there are persistent systemic vascular-metabolic, inflammatory, immune, and rheological disorders, etc.
To correct the latter, in addition to the therapeutic complex we have chosen L-arginine, L-carnitine and quercetin. Numerous clinical studies confirm the effectiveness of L-carnitine [16–19], L-arginine [12, 20, 21] and quercetin [22, 23] in a wide spectrum of diseases, including COVID-19 influenza A virus infection [23].
Additional administration of these pharmacological agents into the therapeutic complex in comparison with the control group of patients, allowed better improvement of the results of treatment both in the aspect of different manifestations of PCS and arthropathy.
The period of administration of L-arginine, L-carnitine and quercetin depended on PCS signs, arthropathy and age of patients. Ellis et al. [21] reported on the need for long-term use of similar agents in patients with PCS and endothelial dysfunction.
Musculoskeletal system lesions with COVID-19 infection and the post-COVID period require further investigation in order to develop new approaches to their treatment and prevention.
Conclusions
Experiencing acute moderate COVID-19 infection contributes to the occurrence of a long-term unfavorable inflammatory, metabolic and autoimmune response, manifested during the post-COVID period by clinical signs of arthralgia, myalgia and arthritis.
In the treatment of musculoskeletal dysfunction, as one of the manifestations of the PCS, it is advisable to include pharmacological agents with multiple action into the rehabilitation complex, in particular, L-arginine, L-carnitine and quercetin.