Bieżący numer
Archiwum
Online first
O czasopiśmie
Redakcja
Rada Naukowa
Wydawca
Standardy etyczne i procedury
Bazy indeksacyjne
Recenzenci
Recenzenci Honorowi
Prenumerata
Kontakt
Najczęściej czytane artykuły
Dla autorów
Opłata za przetwarzanie artykułu (APC)
Książki i Konferencje
Książki
Konferencje
SARS CoV-2/COVID-19
PRACA PRZEGLĄDOWA
Evaluating skin biopsy findings in fibromyalgia: a systematic review
1
Department of Rheumatology and Internal Medicine, Marciniak Lower Silesian Specialist Hospital, Emergency Medicine Center, Wroclaw, Poland
2
Department of Non-Surgical Clinical Sciences, Faculty of Medicine, Wroclaw University of Science and Technology, Poland
Data nadesłania: 14-07-2025
Data ostatniej rewizji: 08-08-2025
Data akceptacji: 26-08-2025
Data publikacji online: 15-01-2026
Autor do korespondencji
Natalia Bejm
Department of Rheumatology and Internal Medicine, Marciniak Lower Silesian Specialist Hospital, Emergency Medicine Center, Wrocław, Poland / Oddział Reumatologii i Chorób Wewnętrznych, Dolnośląski Szpital Specjalistyczny im. T. Marciniaka – Centrum Medycyny Ratunkowej, Wrocław, Polska.
Department of Rheumatology and Internal Medicine, Marciniak Lower Silesian Specialist Hospital, Emergency Medicine Center, Wrocław, Poland / Oddział Reumatologii i Chorób Wewnętrznych, Dolnośląski Szpital Specjalistyczny im. T. Marciniaka – Centrum Medycyny Ratunkowej, Wrocław, Polska.
SŁOWA KLUCZOWE
DZIEDZINY
STRESZCZENIE
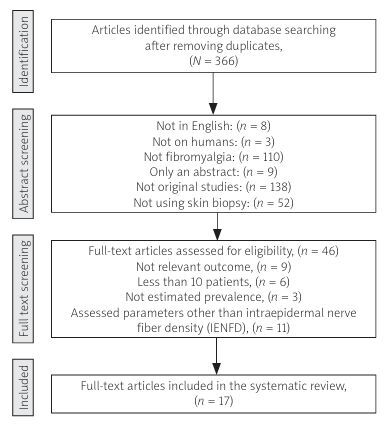
Fibromyalgia syndrome (FMS) is a condition characterized by widespread musculoskeletal pain, often involving a neuropathic component. While pathophysiology remains vague, increasing evidence suggests that small fiber pathology (SFP) may be present in a significant subset of patients, indicating a peripheral nervous system contribution. This systematic review aims to evaluate the utility of skin biopsy as a diagnostic tool for patients with FMS, with special focus on SFP. A comprehensive database search was conducted to identify studies assessing intraepidermal nerve fiber density (IENFD) via skin biopsy in individuals diagnosed with FMS. The included studies demonstrated a reduction in IENFD in a substantial proportion of FMS patients, with reported prevalence ranging widely from 30% to over 85%. Small fiber pathology occurs in a significant proportion of individuals with FMS. Skin biopsy emerges as a valuable diagnostic tool. Further research is needed to better understand the underlying mechanism of SFP in FMS.
REFERENCJE (47)
1.
Clauw DJ. Fibromyalgia: A clinical review. JAMA 2014; 311: 1547–1555, DOI: 10.1001/jama.2014.3266.
2.
Vincent A, Lahr BD, Wolfe F, et al. Prevalence of Fibromyalgia: A Population-Based Study in Olmsted County, Minnesota, Utilizing the Rochester Epidemiology Project. Arthritis Care Res (Hoboken) 2013; 65: 786–792, DOI: 10.1002/acr.21896.
3.
Wolfe F, Ross K, Anderson J, et al. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum 1995; 38: 19–28, DOI: 10.1002/art.1780380104.
4.
McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. Best Pract Res Clin Rheumatol 2007; 21: 403–425, DOI: 10.1016/j.berh.2007.03.003.
5.
Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010; 62: 600–610, DOI: 10.1002/acr.20140.
6.
Wolfe F, Clauw DJ, Fitzcharles MA, et al. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum 2016; 46: 319–329, DOI: 10.1016/j.semarthrit.2016.08.012.
7.
Gyorfi M, Rupp A, Abd-Elsayed A. Fibromyalgia Pathophysiology. Biomedicines 2022; 10: 3070, DOI: 10.3390/biomedicines- 10123070.
8.
Häuser W, Fitzcharles MA. Facts and myths pertaining to fibromyalgia. Dialogues Clin Neurosci 2018; 20: 53–62, DOI: 10.31887/DCNS.2018.20.1/whauser.
9.
Siracusa R, Di Paola R, Cuzzocrea S, Impellizzeri D. Fibromyalgia: Pathogenesis, mechanisms, diagnosis and treatment options update. Int J Mol Sci 2021; 22: 3891. DOI: 10.3390/ijms22083891.
10.
Cagnie B, Coppieters I, Denecker S, et al. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin Arthritis Rheum 2014; 44: 68–75, DOI: 10.1016/j.semarthrit.2014.01.001.
11.
Gracely RH, Geisser ME, Giesecke T, et al. Pain catastrophizing and neural responses to pain among persons with fibromyalgia. Brain 2004; 127 (Pt 4): 835–843, DOI: 10.1093/brain/awh098.
12.
Mendieta D, De la Cruz-Aguilera DL, Barrera-Villalpando MI, et al. IL-8 and IL-6 primarily mediate the inflammatory response in fibromyalgia patients. J Neuroimmunol 2016; 290: 22–25, DOI: 10.1016/j.jneuroim.2015.11.011.
13.
Arnold LM, Fan J, Russell IJ, et al. The Fibromyalgia Family Study: A Genome-Wide Linkage Scan Study. Arthritis Rheum 2013; 65: 1122–1128, DOI: 10.1002/art.37842.
14.
Lee YH, Choi SJ, Ji JD, Song GG. Candidate gene studies of fibromyalgia: a systematic review and meta-analysis. Rheumatol Int 2012; 32: 417–426, DOI: 10.1007/s00296-010-1678-9.
15.
Lacomis D. Small-fiber neuropathy. Muscle Nerve 2002; 26: 173–188, DOI: 10.1002/mus.10181.
16.
Devigili G, Tugnoli V, Penza P, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain 2008; 131: 1912–1925, DOI: 10.1093/brain/awn093.
17.
Terkelsen AJ, Karlsson P, Lauria G, et al. The diagnostic challenge of small fibre neuropathy: clinical presentations, evaluations, and causes. Lancet Neurol 2017; 16: 934–944, DOI: 10.1016/S1474-4422(17)30329-0.
18.
Lauria G, Hsieh ST, Johansson O, et al.; European Federation of Neurological Societies; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010; 17: 903–912, e44–e49, DOI: 10.1111/j.1468-1331.2010.03023.x.
19.
Caro XJ. Immunofluorescent detection of igg at the dermal- epidermal junction in patients with apparent primary fibrositis syndrome. Arthritis Rheum 1984; 27: 1174–1179, DOI: 10.1002/art.1780271014.
20.
Sprott H, Müller A, Heine H. Collagen crosslinks in fibromyalgia. Arthritis Rheum 1997; 40: 1450–1454, DOI: 10.1002/art. 1780400813.
21.
Enestrbm S, Bengtsson A, Frddin T. Dermal IgG Deposits and Increase of Mast Cells in Patients with Fibromyalgia – Relevant Findings or Epiphenomena? Scand J Rheumatol 1997; 26: 308–313, DOI: 10.3109/03009749709105321.
22.
Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013; 154: 2310–2316, DOI: 10.1016/j.pain.2013.06.001.
23.
Üçeyler N, Zeller D, Kahn AK, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013; 136: 1857–1867, DOI: 10.1093/brain/awt053.
24.
Lawson VH, Grewal J, Hackshaw KV, et al. Fibromyalgia syndrome and small fiber, early or mild sensory polyneuropathy. Muscle Nerve 2018; 58: 625–630, DOI: 10.1002/mus.26131.
25.
Boneparth A, Chen S, Horton DB, et al. Epidermal neurite density in skin biopsies from patients with juvenile fibromyalgia. J Rheumatol 2021; 48: 575–578, DOI: 10.3899/jrheum. 200378.
26.
Giannoccaro MP, Donadio V, Incensi A, et al. Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve 2014; 49: 757–759, DOI: 10.1002/mus.24156.
27.
De Tommaso M, Nolano M, Iannone F, et al. Update on laser- evoked potential findings in fibromyalgia patients in light of clinical and skin biopsy features. J Neurol 2014; 261: 461–472, DOI: 10.1007/s00415-013-7211-9.
28.
Kosmidis ML, Koutsogeorgopoulou L, Alexopoulos H, et al. Reduction of Intraepidermal Nerve Fiber Density (IENFD) in the skin biopsies of patients with fibromyalgia: A controlled study. J Neurol Sci 2014; 347: 143–147, DOI: 10.1016/j.jns.2014.09.035.
29.
Leinders M, Doppler K, Klein T, et al. Increased cutaneous miR- let-7d expression correlates with small nerve fiber pathology in patients with fibromyalgia syndrome. Pain 2016; 157: 2493–2503, DOI: 10.1097/j.pain.0000000000000668.
30.
Evdokimov D, Frank J, Klitsch A, et al. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol 2019; 86: 504–516, DOI: 10.1002/ana.25565.
31.
Fasolino A, Di Stefano G, Leone C, et al. Small-fibre pathology has no impact on somatosensory system function in patients with fibromyalgia. Pain 2020; 161: 2385–2393, DOI: 10.1097/ j.pain.0000000000001920.
32.
Evdokimov D, Dinkel P, Frank J, et al. Characterization of dermal skin innervation in fibromyalgia syndrome. PLoS One 2020; 15: e0227674, DOI: 10.1371/journal.pone.0227674.
33.
Vecchio E, Lombardi R, Paolini M, et al. Peripheral and central nervous system correlates in fibromyalgia. Eur J Pain 2020; 24: 1537–1547, DOI: 10.1002/ejp.1607.
34.
Quitadamo SG, Vecchio E, Delussi M, et al. Outcome of small fibre pathology in fibromyalgia: a real life longitudinal observational study. Clin Exp Rheumatol 2023; 41: 1216–1224, DOI: 10.55563/clinexprheumatol/ld0lxn.
35.
Leone C, Galosi E, Esposito N, et al. Small-fibre damage is associated with distinct sensory phenotypes in patients with fibromyalgia and small-fibre neuropathy. Eur J Pain 2023; 27: 163–173, DOI: 10.1002/ejp.2049.
36.
Jänsch S, Evdokimov D, Egenolf N, et al. Distinguishing fibromyal- gia syndrome from small fiber neuropathy: a clinical guide. Pain Rep 2024; 9: e1136. DOI: 10.1097/PR9.0000000000001136.
37.
Feulner B, Gross F, Evdokimov D, et al. Pain and small fiber pathology in men with fibromyalgia syndrome. Pain Rep 2024; 9: e1212, DOI: 10.1097/PR9.0000000000001212.
38.
Falco P, Galosi E, Di Stefano G, et al. Autonomic Small-Fiber Pathology in Patients With Fibromyalgia. J Pain 2024; 25: 64–72, DOI: 10.1016/j.jpain.2023.07.020.
39.
Albrecht PJ, Hou Q, Argoff CE, et al. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: implications for widespread deep tissue pain and fatigue. Pain Med 2013; 14: 895–915, DOI: 10.1111/pme.12139.
40.
Blanco I, Béritze N, Argüelles M, et al. Abnormal overexpression of mastocytes in skin biopsies of fibromyalgia patients. Clin Rheumatol 2010; 29: 1403–1412, DOI: 10.1007/s10067-010-1474-7.
41.
Salemi S, Aeschlimann A, Wollina U, et al. Up-regulation of -opioid receptors and -opioid receptors in the skin of fibromyalgia patients. Arthritis Rheum 2007; 56: 2464–2466, DOI: 10.1002/art.22735.
42.
Üçeyler N, Kewenig S, Kafke W, et al. Skin cytokine expression in patients with fibromyalgia syndrome is not different from controls. BMC Neurol 2014; 14: 185. DOI: 10.1186/s12883-014-0185-0.
43.
Salemi S, Rethage J, Wolina U, et al. Detection of interleukin 1b(IL-1beta), IL-6, and tumor necrosis factor-alpha in skin of patients with fibromyalgia. J Rheumatol 2003; 30: 146–150.
44.
Kim SH, Kim DH, Oh DH, Clauw DJ. Characteristic electron microscopic findings in the skin of patients with fibromyalgia – preliminary study. Clin Rheumatol 2008; 27: 407–411, DOI: 10.1007/s10067-007-0807-7.
45.
Sánchez-Domínguez B, Bullón P, Román-Malo L, et al. Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with Fibromyalgia. Mitochondrion 2015; 21: 69–75, DOI: 10.1016/j.mito.2015.01.010.
46.
Metyas S, Chen C, Quismorio A, et al. Improvement of Nerve Fiber Density in Fibromyalgia Patients Treated with IVIg. Curr Rheumatol Rev 2020; 16: 280–284, DOI: 10.2174/1573397115666191106120622.
47.
Gentile E, Quitadamo SG, Clemente L, et al. A multicomponent physical activity home-based intervention for fibromyalgia patients: effects on clinical and skin biopsy features. Clin Exp Rheumatol 2024; 42: 1156–1163. DOI: 10.55563/clinexprheumatol/iukp4c.
Copyright: © Narodowy Instytut Geriatrii, Reumatologii i Rehabilitacji w Warszawie. This is an Open Access journal, all articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) License (https://creativecommons.org/licenses/by-nc-sa/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, provided the original work is properly cited and states its license.
Udostępnij
Przetwarzamy dane osobowe zbierane podczas odwiedzania serwisu. Realizacja funkcji pozyskiwania informacji o użytkownikach i ich zachowaniu odbywa się poprzez dobrowolnie wprowadzone w formularzach informacje oraz zapisywanie w urządzeniach końcowych plików cookies (tzw. ciasteczka). Dane, w tym pliki cookies, wykorzystywane są w celu realizacji usług, zapewnienia wygodnego korzystania ze strony oraz w celu monitorowania ruchu zgodnie z Polityką prywatności. Dane są także zbierane i przetwarzane przez narzędzie Google Analytics (więcej).
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.
Możesz zmienić ustawienia cookies w swojej przeglądarce. Ograniczenie stosowania plików cookies w konfiguracji przeglądarki może wpłynąć na niektóre funkcjonalności dostępne na stronie.