Current issue
Archive
Online first
About the Journal
Editorial Office
Editorial Board
Publisher
Editorial Policies
Ethical standards and procedures
Abstracting and indexing
Reviewers
Honorary Reviewers
Subscription
Contact
Most read articles
Instructions for authors
Article processing charge (APC)
Books and Events
Books
Events
GERIATRICS / ORIGINAL PAPER
Low muscle mass and body composition analysis in a group of postmenopausal women affected by primary Sjögren’s syndrome
1
Department of Medicine, Surgery and Neurosciences, Rheumatology Unit, University of Siena, Italy
Submission date: 2021-02-03
Final revision date: 2021-04-21
Acceptance date: 2021-05-16
Online publication date: 2021-06-30
Publication date: 2021-07-16
Reumatologia 2021;59(3):153-160
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Sarcopenia is the pathological reduction of skeletal muscle mass and strength. This condition is often underestimated in clinical practice, particularly in connective tissue diseases. The purpose of this study is to evaluate the prevalence of low muscle mass in primary Sjögren’s syndrome (pSS) and to explore the relationships linking muscles and bone tissue.
Material and methods:
Twenty-eight postmenopausal pSS patients were matched with 30 healthy controls and their body composition analysis was performed by dual-energy X-ray absorptiometry to investigate for sarcopenia considering appendicular lean mass (ALM) and the skeletal muscle mass index (SMI) as references. Bone mineral density analysis of lumbar spine (L1–L4), whole femur, femoral neck and whole body was also performed. Linear regression was used to assess the relationship between body composition and bone mineralization.
Results:
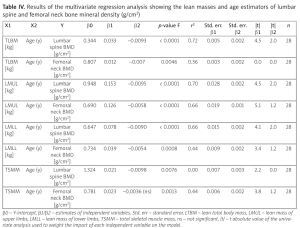
Low muscle mass was significantly higher in the pSS group compared to controls whether expressed as ALM, SMI [odds ratio (OR) = 18.40, confidence interval (CI): 4.84–72.08, p < 0.0001] or considering total body lean masses. Lean masses appeared to be the best estimators of bone mineralization: total lean body mass (TLBM) lumbar spine R2 = 0.72, p < 0.0001; TLBM femoral neck R2 = 0.36, p < 0.004; lean mass of upper limbs lumbar spine R2 = 0.70, p < 0.0001; femoral neck R2 = 0.66; lean mass of lower limbs lumbar spine R2 = 0.66, p < 0.0001; femoral neck R2 = 0.44, p = 0.008). Primary Sjögren’s syndrome patients had a significantly higher android/gynoid fat ratio compared to controls.
Conclusions:
Female pSS patients have lower muscle mass compared to healthy controls and are exposed to a higher risk of developing sarcopenia than healthy subjects. Our research demonstrates that the amount of lean tissue is the main predictor of bone mineralization in pSS.
Sarcopenia is the pathological reduction of skeletal muscle mass and strength. This condition is often underestimated in clinical practice, particularly in connective tissue diseases. The purpose of this study is to evaluate the prevalence of low muscle mass in primary Sjögren’s syndrome (pSS) and to explore the relationships linking muscles and bone tissue.
Material and methods:
Twenty-eight postmenopausal pSS patients were matched with 30 healthy controls and their body composition analysis was performed by dual-energy X-ray absorptiometry to investigate for sarcopenia considering appendicular lean mass (ALM) and the skeletal muscle mass index (SMI) as references. Bone mineral density analysis of lumbar spine (L1–L4), whole femur, femoral neck and whole body was also performed. Linear regression was used to assess the relationship between body composition and bone mineralization.
Results:
Low muscle mass was significantly higher in the pSS group compared to controls whether expressed as ALM, SMI [odds ratio (OR) = 18.40, confidence interval (CI): 4.84–72.08, p < 0.0001] or considering total body lean masses. Lean masses appeared to be the best estimators of bone mineralization: total lean body mass (TLBM) lumbar spine R2 = 0.72, p < 0.0001; TLBM femoral neck R2 = 0.36, p < 0.004; lean mass of upper limbs lumbar spine R2 = 0.70, p < 0.0001; femoral neck R2 = 0.66; lean mass of lower limbs lumbar spine R2 = 0.66, p < 0.0001; femoral neck R2 = 0.44, p = 0.008). Primary Sjögren’s syndrome patients had a significantly higher android/gynoid fat ratio compared to controls.
Conclusions:
Female pSS patients have lower muscle mass compared to healthy controls and are exposed to a higher risk of developing sarcopenia than healthy subjects. Our research demonstrates that the amount of lean tissue is the main predictor of bone mineralization in pSS.
REFERENCES (31)
1.
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. European Working Group On Sarcopenia In Older People 2 (EWGSOP2), and the EXTENDED GROUP FOR EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31, DOI: 10.1093/ageing/afy169.
2.
Doğan SC, Hizmetli S, Hayta E, et al. Sarcopenia in women with rheumatoid arthritis. Eur J Rheumatol 2015; 2: 57–61, DOI: 10.5152/eurjrheum.2015.0038.
3.
Corallo C, Fioravanti A, Tenti S, et al. Sarcopenia in systemic sclerosis: the impact of nutritional, clinical, and laboratory features. Rheumatol Int 2019; 39: 1767–1777, DOI: 10.1007/s00296-019-04401-w.
4.
Korkmaz M, Eyigor S. Association between sarcopenia and rheumatological diseases. World J Rheumatol 2019; 9: 1–8, DOI: 10.5499/wjr.v9.i1.1.
5.
Ngeuleu A, Allali F, Medrare L, et al. Sarcopenia in rheumatoid arthritis: prevalence, influence of disease activity and associated factors. Rheumatol Int 2017; 37: 1015–1020, DOI: 10.1007/s00296-017-3665-x.
6.
Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol 2017; 69: 35–45, DOI: 10.1002/art.39859.
7.
Guevara-Gutiérrez E, Tlacuilo-Parra A, Minjares-Padilla LM. Minor salivary gland punch biopsy for evaluation of Sjögren’s syndrome. J Clin Rheumatol 2001; 7: 401–402, DOI: 10.1097/ 00124743-200112000-00010.
8.
Baumgartner R, Koehler K, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998; 147: 755–763, DOI: 10.1093/oxfordjournals.aje.a009520 [erratum: Am J Epidemiol 1999; 149: 1161].
9.
Kim KM, Jang HC, Lim S. Differences among skeletal muscle mass indices derived from height-, weight-, and body mass index-adjusted models in assessing sarcopenia. Korean J Intern Med 2016; 31: 643–650, DOI: 10.3904/kjim.2016.015.
10.
Montero-Fernández N, Serra-Rexach JA. Role of exercise on sarcopenia in the elderly. Eur J Phys Rehabil Med 2013; 49: 131–143.
11.
Bano G, Trevisan C, Carraro S, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas 2017; 96: 10–15, DOI: 10.1016/j.maturitas.2016.11.006.
12.
Kim MK, Baek KH, Song KH, et al. Vitamin D deficiency is associated with sarcopenia in older Koreans, regardless of obesity: the fourth Korea National Health and Nutrition Examination Surveys (KNHANES IV) 2009. J Clin Endocrinol Metab 2011; 96: 3250–3256, DOI: 10.1210/jc.2011-1602.
13.
Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol, 2016 229: R7–81, DOI: 10.1530/JOE-15-0533.
14.
Kelly OJ, Gilman JC, Kim Y, Ilich JZ. Long-chain polyunsaturated fatty acids may mutually benefit both obesity and osteoporosis. Nutr Res 2013; 33: 521–533, DOI: 10.1016/j.nutres. 2013.04.012.
15.
Argyropoulou OD, Valentini E, Ferro F, et al. One year in review 2018: Sjögren’s syndrome. Clin Exp Rheumatol 2018; 36 (Suppl 112): 14–26.
16.
Tagliaferri C, Wittrant Y, Davicco MJ, et al. Muscle and bone, two interconnected tissues. Ageing Res Rev 2015; 21: 55–70, DOI: 10.1016/j.arr.2015.03.002.
17.
Naot D, Cornish J. Cytokines and hormones that contribute to the positive association between fat and bone. Front Endocrinol (Lausanne) 2014; 5: 70, DOI: 10.3389/fendo.2014.00070.
18.
Trayhurn P, Drevon CA, Eckel J. Secreted proteins from adipose tissue and skeletal muscle – adipokines, myokines and adipose/muscle cross-talk. Arch Physiol Biochem 2011; 117: 47–56, DOI: 10.3109/13813455.2010.535835.
19.
Karpe F. Insulin resistance by adiponectin deficiency: is the action in skeletal muscle. Diabetes 2013; 62: 701–702, DOI: 10.2337/db12-1519.
20.
Bogl LH, Latvala A, Kaprio J, et al. An investigation into the relationship between soft tissue body composition and bone mineral density in a young adult twin sample. J Bone Miner Res 2011; 26: 79–87, DOI: 10.1002/jbmr.192.
21.
Phu S, Boersma D, Duque G. Exercise and sarcopenia. J Clin Densitom 2015; 18: 488–492, DOI: 10.1016/j.jocd.2015.04.011.
22.
Dufour AB, Hannan MT, Murabito JM, et al. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: the Framingham Study. J Gerontol A Biol Sci Med Sci 2013; 68: 168–174, DOI: 10.1093/gerona/gls109.
23.
Baumgartner RN, Wayne SJ, Waters DL, et al. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res 2004; 12: 1995–2004, DOI: 10.1038/oby.2004.250.
24.
Lai EL, Huang WN, Chen HH, et al. Ten-year fracture risk by FRAX and osteoporotic fractures in patients with systemic autoimmune diseases. Lupus 2019; 28: 945–953, DOI: 10.1177/ 0961203319855122.
25.
Yu R, Leung J, Woo J. Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. J Am Med Dir Assoc 2014, DOI: 10.1016/j.jamda.2014.07.011.
26.
Tournadre A, Pereira B, Dutheil F, et al. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J Cachexia Sarcopenia Muscle 2017; 8: 639–646, DOI: 10.1002/jcsm.12189.
27.
Kerekes G, Nurmohamed MT, González-Gay MA, et al. Rheumatoid arthritis and metabolic syndrome. Nat Rev Rheumatol 2014; 10: 691–696, DOI: 10.1038/nrrheum.2014.121.
28.
Gremese E, Tolusso B, Gigante MR, Ferraccioli G. Obesity as a risk and severity factor in rheumatic diseases (autoimmune chronic inflammatory diseases). Front Immunol 2014; 5: 576, DOI: 10.3389/fimmu.2014.00576.
29.
Lazzerini PE, Capecchi PL, Bertolozzi I, et al. Marked QTc prolongation and torsades de pointes in patients with chronic inflammatory arthritis. Front Cardiovasc Med 2016; 3: 31, DOI: 10.3389/fcvm.2016.00031.
30.
Samsell L, Regier M, Walton C, Cottrell L. Importance of Android/gynoid fat ratio in predicting metabolic and cardiovascular disease risk in normal weight as well as overweight and obese children. J Obes 2014; 2014: 846578, DOI: 10.1155/2014/846578.
31.
Gravani F, Papadaki I, Antypa E, et al. Subclinical atherosclerosis and impaired bone health in patients with primary Sjogren’s syndrome: prevalence, clinical and laboratory associations. Arthritis Res Ther 2015; 17: 99, DOI: 10.1186/s13075-015-0613-6.
Copyright: © Narodowy Instytut Geriatrii, Reumatologii i Rehabilitacji w Warszawie. This is an Open Access journal, all articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0) License (https://creativecommons.org/licenses/by-nc-sa/4.0/), allowing third parties to copy and redistribute the material in any medium or format and to remix, transform, and build upon the material, provided the original work is properly cited and states its license.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.