Introduction
Proper nutritional status profoundly impacts functional and clinical outcomes, making achieving and maintaining adequate nutrition at every stage of life essential. It supports physical and mental development, enhances immune function, and prevents health complications. Despite its importance, nutritional disorders and nutrition-related conditions are often underestimated in clinical practice, leading to significant complications. These include functional impairment, prolonged treatment durations, extended hospital stays, impaired wound healing, increased infection rates, and reduced quality of life. These factors contribute to declining general health and increased healthcare costs [1].
Systemic sclerosis (SSc) is a chronic disease that significantly impacts nutritional status. This connective tissue disorder is characterized by vasculopathy, immune system activation, and fibroblast dysfunction. These processes, driven by genetic and environmental factors, result in a wide range of clinical manifestations, including fibrosis of the gastrointestinal tract (GI), lungs, and skin and chronic inflammation, which can severely impair nutritional status [2, 3].
Nutritional disorders and related conditions include malnutrition, sarcopenia, frailty, overweight and obesity, micronutrient deficiencies, and refeeding syndrome [4]. Addressing these conditions requires comprehensive nutritional care, which involves several critical steps: screening for malnutrition risk, conducting thorough nutritional assessments, implementing appropriate diagnostic procedures, developing a personalized nutritional care plan, and continuously monitoring and evaluating the effectiveness of nutritional interventions. This scheme applies to every patient with inadequate nutritional status, regardless of its initial cause [4].
Malnutrition risk screening
Risk screening is an instrument that rapidly and simply evaluates whether the patient is at risk of becoming malnourished. It should be performed by validated tools, which are usually a combination of the history of weight loss over time, presence of anorexia and nausea, reduction of food intake, disease severity, and measurements such as body weight, height, and body mass index (BMI) [5]. The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends several screening tools, which include the Nutritional Risk Screening (NRS 2002), Mini Nutritional Assessment (MNA), and the Malnutrition Universal Screening Tool (MUST) [6]. From the above, only the MUST has been studied in the SSc group. It is a combination of scores from BMI, unplanned weight loss in the past 3–6 months, and the acute disease effect (absence of nutritional intake for more than five days), where a score of at least two points means a high risk of malnutrition. In one study conducted by the Canadian Scleroderma Research Group (CSRG), the mean MUST score was 0.5, but 17.4% of SSc patients showed a high risk of malnutrition (MUST ≥ 2), which should be treated [7]. Other studies have indicated that the percentage of patients with a MUST score of ≥ 2 varies between 6% and 38.3% [7, 8–23]. This large variation in the frequency of malnutrition assessed by the MUST score results from the selection of patients. In studies recruiting inpatients with more severe clinical states, the percentage was higher.
Another screening tool used in SSc is Subjective Global Assessment (SGA). It is a more prognostic instrument and has been shown to have predictive validity in observational studies. The SGA is based on clinical history (weight change in the last 6 months, change in dietary intake, gastrointestinal symptoms, functional capacity, disease in relation to requirements) and clinical examination (the loss of subcutaneous fat and muscles, edema, and ascites) [24]. Assessing the possibility of malnutrition in SSc using the SGA tool shows that its risk can be as high as 50% [8]. Table I shows data on the risk screening tools discussed and malnutrition criteria used [25–35].
Table I
Risk screening tools and criteria for malnutrition used in SSc patients
| First author and year | Country | SSc patient population | Criteria for malnutrition and its risk | Prevalence of malnutrition and its risk [%] |
|---|---|---|---|---|
| Baron et al. 2009 [7] | Canada | 586 | MUST = 1 MUST ≥ 2 | 12.5 17.4 |
| Caporali et al. 2012 [25] | Italy | 160 | BMI < 20 kg/m² and/or 6-month WL ≥ 10% | 15 |
| Murtaugh et al. 2013 [8] | USA | 24 | MUST = 1 MUST ≥ 2 SGA B and C | 8.3 29.2 50 |
| Ortiz-Santamaria et al. 2014 [9] | Spain | 72 | MUST ≥ 1 | 12.5 |
| Cereda et al. 2014 [10] | Italy | 160 | MUST = 1 MUST ≥ 2 | 30 24.4 |
| Krause et al. 2014 [26] | Germany | 124 | PhA values | 55.7 |
| Rosato et al. 2014 [27] | Italy | 94 | BMI < 20 kg/m² | 19 |
| Spanjer et al. 2017 [28] | Netherlands | 72 | ESPEN | 8.3 |
| Caimmi et al. 2018 [11] | Italy | 141 | MUST = 1 MUST ≥ 2 ESPEN | 7.8 12.8 9.2 |
| Dupont et al. 2018 [12] | France | 82 | MUST ≥ 2 French HAS | 15 17 |
| Preis et al. 2018 [13] | Germany | 129 | MUST ≥ 2 | 10.9 |
| Corallo et al. 2019 [29] | Italy | 62 | ESPEN | 19 |
| Wojteczek et al. 2019 [30] | Poland | 56 | ESPEN GLIM | 17.9 62.5 |
| Türk et al. 2020 [14] | Turkey | 98 | MUST = 1 MUST ≥ 2 | 15.3 23.5 |
| Yalcinkaya et al. 2020 [15] | Turkey | 114 | MUST = 1 MUST ≥ 2 | 9 6 |
| Molfino et al. 2020 [16] | Italy | 64 | MUST = 1 MUST ≥ 2 | 12.5 26.5 |
| Paolino et al. 2020 [31] | Italy | 36 | FFMI < 15 kg/m2 in women and < 17 kg/m2 in men | 36.1 |
| Hvas et al. 2020 [17] | UK | 168 | MUST = 1 MUST ≥ 2 | 14 12 |
| Pinheiro et al. 2020 [18] | Brazil | 98 | MUST = 1 MUST ≥ 2 | 8 17 |
| Mękal et al. 2021 [32] | Poland | 32 | BMI < 20 kg/m² | 6.3 |
| Rosato et al. 2021 [19] | Italy | 102 | MUST = 1 MUST ≥ 2 ESPEN GLIM | 12.7 17.7 8.8 16.6 |
| Burlui et al. 2021 [20] | Italy | 42 | MUST = 1 MUST ≥ 2 | 11.9 14.29 |
| Rosato et al. 2022 [21] | Italy | 69 | MUST = 1 MUST ≥ 2 ESPEN GLIM | 11.6 14.5 11.6 23.2 |
| Rosato et al. 2023 [33] | Italy | 101 | GLIM | 21.8 |
| Rosato et al. 2023 [22] | Italy | 104 | MUST = 1 MUST ≥ 2 GLIM | 10.6 20.2 20.2 |
| Rivet et al. 2023 [23] | France | 120 | MUST = 1 MUST ≥ 2 French PNDS 2020 | 20 38.3 59.2 |
| Fairley et al. 2024 [34] | Australia | 1903 | GLIM | 43 |
| Wojteczek et al. 2024 [35] | Poland | 56 | Pre-cachexia 7-point SGA and/or albumin < 34 g/l | 8.9 19.7 |
[i] BMI – body mass index, ESPEN – European Society for Clinical Nutrition and Metabolism, FFMI – fat-free mass index, French HAS – French National Authority for Health, French PNDS 2020 – French National Diagnosis and Care Protocol 2020, GLIM – Global Leadership Initiative on Malnutrition, MUST – Malnutrition Universal Screening Tool, PhA – phase angle, SGA – Subjective Global Assessment, WL – weight loss.
Nutritional assessment
All of the patients who are at risk of nutrition disorders should undergo proper nutritional assessment, which is an essential part of making a diagnosis of malnutrition and, further, its treatment. Proper nutritional assessment consists of several steps, such as anthropometric measurements, body composition evaluation, muscle strength and physical functioning estimation, quality of life, biochemical tests, and disease activity and severity [4].
Anthropometric measurements and body composition
Basic anthropometric measurements are body weight, height, BMI, triceps skinfold thickness, and mid-arm (MAC) or calf circumferences. In one study, MAC was below the 5th centile for the general population in 17% and TSF in 22% of SSc patients [17]. No significant differences were found between SSc patients and the healthy population in the waist-to-hip ratio and arm circumference in the Molfino study, but a smaller hip circumference was noted in the SSc population in other analyses [14, 35]. Body mass index, which is defined as a person’s weight in kilograms divided by the square of their height in meters, is a commonly used parameter. In the first reports concerning nutritional disorders in SSc, malnutrition was defined as BMI < 20 kg/m², and its incidence ranged from 6.3% to 19% [25, 27, 32]. Following ESPEN 2015 criteria, diagnosis of malnutrition can be made only when BMI is < 18.5 kg/m². In studies where the assessment was based on this value, underweight ranged from 3.1% to 18% [10, 13, 15, 26, 30, 36]. Body mass index is simple and easy to apply in clinical practice, but it can be misleading due to the growing prevalence of overweight and obesity. Attention is drawn to the possibility of undernutrition or sarcopenia among patients with higher BMI values with low fat-free mass (FFM), which implies that malnutrition can be compensated by high fat mass (FM) [37].
Body composition can be evaluated by different methods, but bioelectric impedance analysis (BIA) is the most common. Other less commonly utilized tools include computed tomography, magnetic resonance imaging, and dual-energy X-ray absorptiometry (DXA). Bioelectric impedance analysis is an easy, non-invasive, quick, bedside method. This method assesses body impedance by applying an electric current passing through the body. The electrical impedance consists of two parts: reactance measuring body cell mass (BCM) and resistance checking total body water. Fat-free mass includes bone minerals and BCM [38]. Low fat-free mass index (FFMI) in patients with SSc often ranges from 20.8% to 28.2% [16, 28, 39]. In one study where the diagnosis of malnutrition was based only on low FFMI, defined as < 15 kg/m2 in women and < 17 kg/m2 in men, its prevalence was 36.1%. Those SSc patients had significantly lower serum albumin and hemoglobin concentrations and lower bone mineral density in the lumbar spine [31]. Moreover, low FFMI is negatively correlated with disease activity and severity and is associated with gastrointestinal involvement (distention/bloating) and disease duration [19, 21]. Another parameter measured by BIA that correlates with nutritional status is the phase angle (PhA). The PhA is considered an indicator of cellular health correlated with nutritional status. Malnutrition assessed by this parameter can affect 44.3% to 55.7% of SSc patients [28, 39]. It has been confirmed that BIA is a good and suitable device for assessing body composition in SSc patients and has been validated by DXA [28]. The DXA can give additional information about body composition. This tool can assess FM and bone mineral content, except FFM. In one study, all body composition compartments in the SSc group were significantly lower than in healthy controls [36].
Estimation of muscle strength, physical functioning and quality of life
The main tools for evaluating physical function are hand grip strength (HGS), measured by a hand-held dynamometer, and gait speed of the chair rise test. Both of them are appliances that are easy to perform. From the above, only the HGS has been studied in the SSc group. Lower HGS at hospital admission correlates with longer hospitalization time and SGA score/category. The purpose of the HGS evaluation is wide and includes the diagnosis of diseases, evaluation, and monitoring of muscle strength during its treatment and rehabilitation. In SSc patients, HGS can be diminished by up to 95%, which correlates with FFMI [30].
Finger mobility is one of the physical functioning factors that can significantly impact nutrition in SSc. Studies have confirmed that a reduced finger interincisal distance is associated with an increased risk of malnutrition [14, 23].
Other suitable tools for assessing physical functioning and quality of life include such scores as the Short Physical Performance Battery, the International Physical Activity Questionnaire (IPAQ), the Short Form-36 Health Survey (SF-36 QoL), the Health-Related Quality-of-Life (HRQoL) and the Scleroderma Health Assessment Questionnaire (SHAQ). It has been confirmed that physical activity is lower in malnourished SSc subjects than in non-malnourished subjects [11, 34]. Undernutrition also affects the quality of life, and almost all of the scores in SF-36 QoL have been shown to be significantly lower in comparison with well-nourished patients. The affected components were physical functioning, physical role, vitality, and social functioning. Only self-reported general health and bodily pain were not influenced by dietary deficiencies. In the same population, SHAQ, which measures patients’ disease-related impairment, was higher in malnourished cases [13]. Moreover, anxiety and depression were noted in patients with higher MUST scores [9, 14].
Biochemical tests
Visceral protein levels are used in practice as markers of nutritional status. However, their reduction is rarely caused by improper nutrition as currently inflammation is acknowledged as the main reason for their reduction in serum. That is the rationale for not using albumin or prealbumin levels alone without additional parameters as tools for screening or diagnosing malnutrition [40]. Their levels can be used as markers of improvement in disease-related malnutrition without inflammation during nutritional treatment. In the case of disease-related malnutrition with inflammation, termed cachexia, low albumin levels with elevated inflammatory parameters indicate mainly a level of catabolism and can constitute a prognostic factor [4]. However, it seems that prealbumin is a more sensitive parameter correlated with malnutrition, being significantly lower in malnourished SSc patients [25]. Some studies have not shown such a relationship for albumin [27, 34]. Still, recent research based on a large population of SSc patients reported statistically significant hypoalbuminemia in malnourished patients with a diagnosis based on GLIM (Global Leadership Initiative on Malnutrition) criteria [35]. Furthermore, it was confirmed that in SSc patients, low serum prealbumin concentration is associated with higher mortality regardless of other significant risk factors, including comorbidities and organ involvement [41]. In relation to inflammatory parameters such as C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR), the relationship between their high levels and malnutrition depends on the screening tool used to diagnose improper nutritional status. There was a correlation between malnutrition assessed by phase angle, GLIM, and 7-point SGA (Subjective Global Assessment) and high ESR values. Still, such a relationship with inflammatory parameters was not proven in the cases in which the MUST score was used [8, 11, 25, 26, 34, 35]. Among other parameters, low hemoglobin levels were associated with malnutrition [7, 11–13, 31, 34, 35]. In a study with a large patient population assessing malnutrition using the GLIM criteria, malnutrition was more common in patients with positive p/RNA polymerase III antibodies [34]. However, a different pattern emerged in the Krause study, where lower PhA values were associated with the presence of p/PM-Scl 75 antibodies [26].
Assessment of disease activity and severityseverity of the disease and organ involvement
Assessing disease activity in SSc is more challenging than in other rheumatic diseases. In SSc, two aspects of the disease play a role in the whole picture of the disease. In the active phase, there can occur potentially reversible changes. That stage is more difficult to assess in limited SSc (lSSc) than in diffuse SSc (dSSc), where the course of active disease is more hidden and slower. Taking into account this fact, the most acknowledged disease activity index proposed by the European Scleroderma Study Group (EScSG), Valentini’s Scleroderma Disease Activity Score (SDAS), has three variances: one for SSc as a whole (whole series index), one for diffuse cutaneous SSc (dcSSc), and one for limited cutaneous SSc (lcSSc) [42]. The SDAS, where the maximum score for all three indices is 10, includes weighted components such as total skin score > 20, scleroderma, digital necrosis, arthritis, level of ESR > 30 mm/h, total lung capacity < 80% of predicted normal values, hypocomplementemia and changes in the past month in cardiopulmonary, skin, vascular and articular/muscular symptoms. The SDAS did not demonstrate any correlation with malnutrition, as assessed by BMI < 20 kg/m², nor with malnutrition according to the ESPEN criteria [19, 27]. However, a low PhA was identified as a marker of disease activity. Additionally, malnourished patients assessed using the GLIM criteria scored higher on the SDAS [19, 43].
Another aspect of SSc is organ damage, usually connected with irreversible fibrosis. In the Medsger severity score, known as Disease Severity Score (DSS), nine organ systems (general, peripheral vascular, skin, joint/tendon, muscle, GI, lung, heart, kidney) are assessed on a scale from 0 (no organ involvement) to 4 (end-stage disease) [44].
The general DSS was significantly higher in SSc patients identified as malnourished according to PhA, French National Authority for Health (French HAS), and GLIM criteria [12, 19, 43]. Undernutrition, as assessed by the ESPEN criteria in the Caimmi et al. [11] study, was also associated with worse general DSS and lung DSS. However, the Rosato study did not demonstrate such an association [11, 19].
In the first reports concerning the association of GI involvement, anorexia was predictive of a higher MUST score [7]. Later studies found no connections between GI symptoms and PhA, low BMI, or malnutrition based on the French HAS [12, 25, 26]. Other studies used the University of California Los Angeles Scleroderma Clinical Trials Consortium Gastrointestinal Tract score (UCLA SCTC GIT 2.0 Score), a validated tool for assessing GI symptoms and their impact on mental and social health. Severe GI disease assessed by this score was shown to be related to the risk for malnutrition [14, 15], also assessed by ESPEN criteria [11] and GLIM criteria [34].
Other data indicate the connection between lung involvement and undernutrition. Patients at high risk for malnutrition had interstitial lung disease and limited forced vital capacity (FVC) and diffusing capacity for carbon monoxide (DLCO) more frequently than well-nourished patients [12, 14, 15]. In malnourished patients assessed by PhA and ESPEN criteria, lower FVC values were noted [11, 26].
Cardiac involvement was observed more often in SSc patients with a higher risk of malnutrition [13, 23]. Moreover, MUST score showed a significant positive correlation with left ventricular mass index (LVMI) and FFMI, indicating that cardiac mass may be one of the tools of nutritional assessment [16].
Major vascular complications, including digital ulcers, pulmonary arterial hypertension, and scleroderma renal crisis, have been associated with decreased FFMI and PhA values [39]. Patients with reduced PhA were found to have a relative risk of 10.1 for developing new digital ulcers [39]. Pulmonary arterial hypertension has been linked to malnutrition, as evaluated by the GLIM criteria [34]. Additionally, a low capillary number (≤ 6/mm) and the presence of a late scleroderma pattern in capillaroscopy were linked to a medium to high risk of malnutrition, as defined by the MUST criteria [15].
Diagnosis of malnutrition
Malnutrition, which remains an underestimated problem, has been described as “a state resulting from lack of uptake or intake of nutrition leading to altered body composition (decreased fat-free mass) and body cell mass leading to diminished physical and mental function and impaired clinical outcome from disease” [37]. For many years this part of the assessment posed a problem due to the lack of consensus for diagnostic criteria.
Malnutrition risk assessment tools, such as the MUST, were often used to establish the diagnosis of malnutrition in SSc patients. One of the first validated tools used in the SSc population, a semi-gold standard for assessing malnutrition, was the SGA. Malnutrition assessed by this parameter was as high as 50% [8]. A modification of this scale known as the 7-point SGA is more sensitive to changes in nutrition than the conventional SGA [45]. With this tool, the prevalence of malnutrition in patients with SSc reaches 20% [35]. In two French studies, alternative tools were employed, including the French National Authority for Health (French HAS) and the French National Diagnosis and Care Protocol 2020 (French PNDS) recommendations for SSc, which define malnutrition by criteria such as weight loss of 5% within one month or 10% within 6 months, a BMI < 21 kg/m², or albuminemia < 35 g/l. Using these criteria, the prevalence of malnutrition was almost 60% [12, 23].
In 2015, the ESPEN introduced new criteria for the diagnosis of malnutrition:
BMI < 18.5 kg/m² or
unintentional weight loss > 10% over an indefinite period, or > 5% in the previous 3 months combined with a BMI < 20 kg/m² if 70 years of age, or < 22 kg/m² if ≥ 70 years of age or
unintentional weight loss > 10% over an indefinite period or > 5% in the previous 3 months combined with FFMI < 15 kg/m2 in women and 17 kg/m2 in men [37].
Based on those criteria, malnutrition in the SSc population ranges from 8.3% to 19% [11, 19, 21, 28–30].
In 2019, the GLIM published other criteria for malnutrition, trying to standardize them globally. The criteria consist of three phenotypic ones:
unintentional weight loss > 5% within the past 6 months or > 10% beyond 6 months,
low BMI < 20 kg/m² if < 70 years (in Asia < 18.5 kg/m²) or < 22 kg/m² if > 70 years (in Asia < 20 kg/m²),
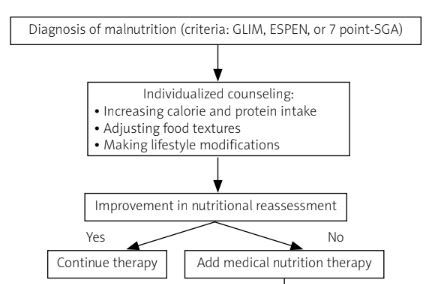
reduced muscle mass, and two etiologic ones: reduced food intake or assimilation, inflammation or disease burden. To diagnose malnutrition, at least one phenotypic and one etiologic criterion should be present [46]. The prevalence of malnutrition using GLIM criteria in the SSc population ranges from 16.6% to 62.5% [19, 21, 22, 30, 34]. Figure 1 shows the diagnostic algorithm for nutritional disorders in systemic sclerosis.
Sarcopenia in systemic sclerosis
Sarcopenia is characterized by a progressive and generalized loss of skeletal muscle mass and its function [47]. It is most commonly associated with aging, though it can also occur due to various conditions earlier in life. The most frequent causes of sarcopenia are nutritional, connected with inactivity, and various diseases [48]. Sarcopenia has evolved from being understood solely as the loss of muscle mass to now encompassing both muscle mass and muscle function (strength and performance). The European Working Group on Sarcopenia in Older People (EWGSOP) has been influential in defining and updating the criteria for sarcopenia. The latest update (EWGSOP2) emphasizes muscle strength, usually measured by grip strength (norms: men < 27 kg, women < 16 kg), as a primary criterion for diagnosing sarcopenia [49]. Muscle mass is considered alongside muscle strength to confirm the diagnosis. Muscle mass is usually assessed by DXA. To standardize cut-offs of results, the lean mass result is adjusted for height (norms: men < 7 kg/m², women < 5.5 kg/m²). Physical performance, such as gait speed or the Timed Up and Go test, can be used to assess the severity of sarcopenia [48]. Diagnosing and treating sarcopenia is important because this condition leads to significant adverse outcomes, including increased risk of falls, functional decline, frailty, and mortality [48]. In SSc, sarcopenia has been increasingly recognized and has a profound impact on the quality of life and overall prognosis. The prevalence of sarcopenia in patients with systemic sclerosis depends on the criteria used for its diagnosis and varies from 10.9% to 42% [29, 50–54]. These figures indicate a significantly higher prevalence than in the general population, which is 10%, suggesting that SSc patients are particularly vulnerable to muscle loss [55]. Table II shows studies of the prevalence of sarcopenia in SSc.
Table II
Studies on the prevalence of sarcopenia in systemic sclerosis
| First author and year | Country | SSc patient population | Criteria for sarcopenia | Prevalence of sarcopenia [%] |
|---|---|---|---|---|
| Caimmi et al. 2018 [11] | Italy | 141 patients | SMI (LMM: ASMI, < 7.26 kg/m² for men and < 5.5 kg/m² for women) | 20.7% |
| Siegert et al. 2018 [50] | Germany | 129 patients | EWGSOP (LMM: ALM/height², < 7.26 kg/m² for men and < 5.5 kg/m² for women, and BMI-stratified HGS cut-off values) | 22.5% |
| Corallo et al. 2019 [29] | Italy | 62 patients | EWGSOP (LMM: RSMI, < 7.26 kg/m² for men and < 5.5 kg/m² for women; HGS, < 30 kg for men and < 20 kg for women) | 42.0% |
| Sari et al. 2020 [51] | Turkey | 93 patients | EWGSOP (LMM: ASMI of < 7.26 kg/m2 for men and < 5.50 kg/m2 for women, and BMI-stratified HGS cut-off values) | 10.7% |
| Paolino et al. 2020 [52] | Italy | 43 patients | EWGSOP (LMM: RSMI, < 7.26 kg/m² for men and < 5.5 kg/m² for women) | 23.26% |
| Hax et al. 2021 [53] | Brazil | 94 patients | EWGSOP2 (LMM: ASMI, < 7.0 kg/m² men and < 5.5 kg/m² women; HGS < 27 kg for men and < 16 kg for women; SPPB ≤ 8) | 15.9% and severe sarcopenia in 5.3% |
| Sangaroon et al. 2022 [54] | Thailand | 180 patients | AWGS (LMM: ASMI, < 7.0 kg/m² for men and < 5.4 kg/m² for women; HGS < 28 kg for men and < 18 kg for women; GS < 1.0 m/s for both men and women) | 22.8% and severe sarcopenia in 73.2% |
[i] ASMI – appendicular skeletal muscle mass index, AWGS – Asian Working Group of Sarcopenia, BMI – body mass index, EWGSOP – European Working Group of Sarcopenia in Older People, GS – gait speed test, HGS – handgrip strength, LMM – low muscle mass, RSMI – relative skeletal muscle mass index, SMI – skeletal muscle mass index, SPPB – Short Physical Performance Battery.
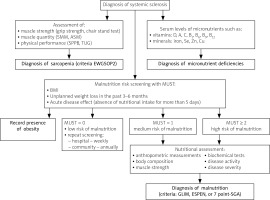
Fig. 1
The diagnostic algorithm for nutritional disorders in systemic sclerosis.
7-point SGA – 7-point Subjective Global Assessment, ASM – appendicular skeletal muscle mass, BMI – body mass index, Cu – cooper, ESPEN – European Society for Clinical Nutrition and Metabolism, EWGSOP2 – European Working Group on Sarcopenia in Older People, GLIM – the Global Leadership Initiative on Malnutrition, MUST – Malnutrition Universal Screening Tool, Se – selenium, SMM – skeletal muscle mass, SPPB – Short Physical Performance Battery, TUG – Timed-Up and Go test, Zn – zinc.
Several factors have been identified as being associated with the development of sarcopenia in SSc patients. Notably, longer disease duration, esophageal involvement, higher modified Rodnan Skin Scores (mRSS), elevated ESR, and higher CRP levels have all been linked to an increased risk of sarcopenia [28, 29, 54]. Additionally, sarcopenic patients demonstrated higher Medsger severity scores for both lung and skin involvement [4]. Pulmonary function, particularly predicted values of DLCO, was lower in sarcopenic patients [11, 29]. Patients with the dcSSc subset had lower relative skeletal muscle mass index (RSMI) and bone mineral density (BMD) values in comparison with limited SSc (lsSSc) patients [36]. A low BMI at disease onset has also been associated with sarcopenia [11, 54]. Moreover, sarcopenia was significantly more prevalent among malnourished patients compared to non-malnourished patients (84.6% vs. 14.1%, p < 0.001) [11]. Capillaroscopy findings further highlighted the link between sarcopenia and microvascular damage. A “late” scleroderma pattern in capillaroscopy was associated with a significantly higher prevalence of sarcopenia compared to other capillaroscopy patterns [29, 52]. Additionally, capillary density was found to be significantly lower in sarcopenic patients compared to non-sarcopenic ones [25]. Moreover, sarcopenia significantly affects the quality of life of SSc patients. Those with sarcopenia had a poorer quality of life in the SF-36 survey, which measures physical and emotional well-being [51, 52].
These findings underscore the importance of closely monitoring SSc patients for signs of sarcopenia, where early detection and intervention could potentially mitigate the impact of sarcopenia on this vulnerable patient population.
Overweight and obesity
Overweight and obesity are characterized by abnormal or excessive fat accumulation. Both nutritional abnormalities are recognized and classified by the BMI. Overweight is classified when BMI falls between 25 and 30 kg/m², while obesity is defined as a BMI of 30 kg/m² or higher [4]. For the Asian population, these thresholds are often adjusted, with overweight typically recognized at a BMI of 23 to 27.5 kg/m² and obesity starting at a BMI of 27.5 kg/m² or higher [56]. Surprisingly, in SSc studies, the rate of overweight ranges from 25.8% to 39.3%, and obesity from 4.8% to 28.1%. Table III shows studies demonstrating the prevalence of overweight and obesity in the SSc population. However, it has to be stressed that the presence of overweight and obesity does not exclude the possibility of malnutrition and/or sarcopenia in this group of patients due to the possibility of low FFMI [57].
Table III
Prevalence of overweight and obesity in SSc population
| First author and year | Country | SSc patient population | Criteria for overweight and obesity | Prevalence of overweight and obesity |
|---|---|---|---|---|
| Marighela et al. 2013 [36] | Brazil | 61 | BMI 25.0–29.9 kg/m² BMI ≥ 30 kg/m² | 34.4% 18.0% |
| Krause et al. 2010 [26] | Germany | 124 | BMI 25.0–29.9 kg/m² BMI ≥ 3 0 kg/m² | 25.8% 4.8% |
| Spanjer et al. 2017 [28] | Netherlands | 72 | BMI 25.0–29.9 kg/m² BMI ≥ 3 0 kg/m² | 27.8% 11.1% |
| Wojteczek et al. 2019 [30] | Poland | 56 | BMI 25.0–29.9 kg/m² BMI ≥ 30 kg/m² | 39.3% 12.5% |
| Paolino et al. 2020 [52] | Italy | 43 | BMI 25.0–29.9 kg/m² BMI ≥ 30 kg/m² | 30.23% 9.3% |
| Mękal et al. 2021 [32] | Poland | 32 | BMI 25.0–29.9 kg/m² BMI ≥ 30 kg/m² | 31.5% 3.1% |
| Fairley et al. 2024 [34] | Australia | 1903 | BMI ≥ 30 kg/m² | 28.1% |
Micronutrient deficiencies
Most of the data about micronutrient deficiency in SSc patients focus on vitamin D. The prevalence of vitamin D deficiency is 25%, and its median level ranges from 13.1 ng/ml to 43.7 ng/ml [58]. Other vitamin deficiencies, such as vitamin B12 (cobalamin), vitamin B9 (folate), vitamin B6 (pyridoxine), vitamin B1 (thiamine), and vitamin C (ascorbic acid), have also been reported [58]. From minerals, low selenium (Se) was associated with cardiac involvement [12].
Nutritional treatment
Nutritional interventions can take various forms, from providing eating support, offering dietary advice, and counseling on food choices and preparation to prescribing therapeutic diets tailored to the patient’s specific needs. Additionally, medical nutrition therapy is a comprehensive approach that includes oral nutritional supplements, enteral tube feeding (enteral nutrition), and parenteral nutrition. The choice of support should be assessed in an individualized way depending on the patient’s status [4]. In studies where nutritional treatment was personalized, it typically involved individualized counseling that focused on increasing calorie and protein intake, adjusting food textures, and making lifestyle modifications to prevent further weight loss [59]. These tailored approaches were also addressed to a broad spectrum of gastrointestinal symptoms. In Doerfler et al.’s study, 6 weeks of that strategy resulted in improvement in the abridged Patient-Generated Subjective Global Assessment (abPG-SGA) and appendicular lean height (ALH) assessed by DXA [60].
In many clinical situations, additional oral nutritional supplements are essential. In the Ortiz-Santamaria et al. [9] study, all malnourished patients assessed with the MUST score (9 out of 72 patients, 12.5%) underwent 12 months of individualized counseling strategy. During the follow-up visits, three patients needed oral nutritional supplements (ONS), 500 ml daily of complete, polymer, and normoproteic normocaloric diet. The treatment resulted in maintaining or increasing body weight [9]. In Krause et al.’s [26] study, all patients with signs of malnutrition, based on PhA measurements, received individual nutritional advice. Of them, 32 needed additional ONS and one parenteral nutrition. After follow-up with a mean period of 14.9 (SD 6.76) months, improvement in PhA was observed [26]. In our study, all malnourished patients assessed by 7-point SGA and/or serum level albumins < 34 g/l (8 out of 56 patients, 19.7%) were given 200 ml of a high-protein supplement containing 250 kcal and 19 g of protein for 3 months. At the follow-up visit, the 7-point SGA, intracellular water (ICW), lean tissue index (LTI), lean tissue mass (LTM), and BCM scores were significantly higher than at baseline [35]. Table IV lists studies on enteral nutrition in SSc patients experiencing malnutrition or at high risk of developing it.
Table IV
Studies on enteral nutrition in SSc patients with a high risk of malnutrition and/or malnutrition
| First author and year | SSc population treated with enteral nutrition | Nutritional intervention | Time of the nutritional therapy | Reported outcomes |
|---|---|---|---|---|
| Ortiz-Santamaria et al. 2014 [9] | 9 malnourished patients of 72 (12.5% with MUST ≥ 1) | Individualized counseling* During follow-up visits, 3 patients were prescribed ONS 500 ml daily of complete, polymer, and normoproteic normocaloric diet (rejected by one patient) | 12 months | Maintaining or increasing body weight |
| Krause et al. 2010 [26] | 69 malnourished patients of 124 (55.7% with PhA < 4.9°) | Individualized counseling* 32 patients were on ONS therapy 1 patient was on PN | 14.9 (SD 6.76) months | Improvement in PhA |
| Doerfler et al. 2017 [60] | 18 patients with GI involvement (100%), of whom 15 were malnourished (83% with abPG-SGA) and 9 sarcopenic (50%) | Individualized counseling* | 6 weeks | Improvement in abPG-SGA and ALH |
| Yalcinkaya et al. 2020 [15] | 8 malnourished patients of 134 (6% with MUST ≥ 2) | Enteral nutrition (no data about kind of therapy) | No data | No data |
| Wojteczek et al. 2024 [35] | 5 pre-cachectic patients of 56 (8.9%) 8 malnourished patients of 56 (8.9% with 7-point SGA < 5 points and/or serum albumin concentration < 34 g/l) | Individualized counseling* High-protein ONS (200 ml once a day, containing 250 kcal and 19 g of protein) | 3 months | In pre-cachectic stabilization in 7-point SGA In malnourished SSc improvement in 7-point SGA and BIA |
* Individualized counseling consisted of increased calorie intake, modified textures, lifestyle modifications.
7-point SGA – 7-point Subjective Global Assessment, abPG-SGA – abridged Patient-Generated Subjective Global Assessment, ALH – appendicular lean height, BIA – bioelectric impedance analysis, GI – gastrointestinal tract, MUST – Malnutrition Universal Screening Tool, ONS – oral nutritional supplement, PhA – phase angle, PN – parenteral nutrition, SD – standard deviation.
Two randomized control studies with the use of probiotics were conducted; however, their effect was evaluated in terms of the symptoms of the GI tract only. In the study by Low et al., 120 days of Vivomixx 1800 billion units/day significantly improved GI reflux assessed in UCLA GIT 2.0. [61]. In the study by Marighela et al., after eight weeks of probiotics (Lacto-pro, Invictus, Rio de Janeiro, Brazil), there was no difference in the UCLA GIT 2.0 score between the placebo and treatment groups [62].
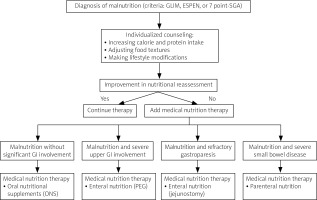
However, when oral nutrition is inadequate in severe cases, enteral or parenteral nutrition is necessary. Enteral feeding via percutaneous endoscopic gastrostomy (PEG) was reported in several cases with severe malnutrition (BMI < 17 kg/m²) and symptoms from the upper GI tract. In those cases, this kind of nutrition improved nutritional status by gaining weight or at least its stabilization [63–65]. In some clinical situations, such as refractory gastroparesis, jejunostomy can be effective [66]. Parenteral nutrition is usually needed when the symptoms of intestinal failure occur. For patients experiencing malabsorption due to small bowel disease, home parenteral nutrition (HPN) can be an option to stabilize or improve nutritional status. In the largest study, where 25 patients with SSc were on HPN treatment, 100% of patients had small bowel dysmotility. All of the patients were given parenteral nutrition via a single-lumen tunneled CVC. During HPN therapy, median BMI increased from 18.5 to 21.3 in 12 months. Eight of the 25 patients were alive at the end of the study period, which was 1990–2012. None of the patients died because of complications of HPN, which were catheter-related bloodstream infection, venous thrombosis, and non-thrombotic CVC occlusion [67]. In Suzon et al.’s study, another additional side effect was pointed out: HPN-related cardiac overload [68]. In other reports on HPN treatment in SSc, which described smaller groups of patients, similar effects were seen. Those results suggest that HPN can be safely and successfully used long term in SSc and should be considered for patients with severe GI involvement who are unable to maintain their nutritional status [68–72]. Figure 2 shows the treatment algorithm for managing malnutrition in systemic sclerosis.
Fig. 2
The treatment algorithm for managing malnutrition in systemic sclerosis.
7-point SGA – 7-point Subjective Global Assessment, ESPEN – European Society for Clinical Nutrition and Metabolism, GI – gastrointestinal, GLIM – Global Leadership Initiative on Malnutrition, PEG – endoscopic gastrostomy, SSc – systemic sclerosis.
Conclusions
Nutritional disorders are prevalent yet underrecognized complications in SSc patients, significantly impacting the course of the disease and patient quality of life. Early detection and individualized nutritional interventions, including enteral and parenteral nutrition in severe cases, are essential for improving outcomes. Regular nutritional assessment and the use of validated screening tools can help guide treatment, ensuring that nutritional support is effectively tailored to the needs of each patient. Further research is needed to optimize these interventions and develop comprehensive care strategies.