Introduction
Osteoarthritis (OA) is a chronic degenerative disease that affects the joints and is characterized by a gradual loss of articular cartilage, subchondral bone remodelling, and inflammation of the joints. Although age, sex, ethnic background, genetics, bone density, oestrogen replacement therapy (in postmenopausal women), diet, obesity, and local biomechanical abnormalities are the most common risk factors for osteoarthritis [1, 2], trauma has also been linked to the onset and progression of this condition.
Post-traumatic osteoarthritis (PTOA), a disorder of the synovium, subchondral bone, and cartilage that affects the entire joint, constitutes approximately 12% of all cases of symptomatic osteoarthritis [3, 4].
The development of PTOA is noted after a joint injury, such as a fracture that occurs within a joint, or an injury to the ligaments or cartilage (including the meniscus) within a joint. These injuries, especially those affecting the lower extremity joints, often occur as a result of trauma or accidents associated with athletic or military activities [5].
While PTOA can occur at any age, it mainly affects young and middle-aged individuals. The pain and functional disability resulting from PTOA not only impair patients’ quality of life but also impose a significant economic burden.
At the molecular level, various factors such as inflammation and cytokine release in the cartilage and synovium, oxidative and nitrosative stress, chondrocyte death, mitochondrial dysfunction, remodelling of subchondral bone, as well as altered biomechanics, can contribute to the degradation of joint tissues, resulting in pain, stiffness, and reduced mobility [5–7].
This article will focus on the peculiarities of PTOA development with a particular emphasis on the role of the systemic inflammatory response and the activation of pro-inflammatory signalling pathways, associated with nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB), mitogen-activated protein kinases (MAPKs), signal transducer and activator of transcription 3 (STAT3), etc.
Unfortunately, there are not any disease-modifying treatments available right now to stop or slow the PTOA course [5, 8]. However, there is increasing evidence suggesting that chondroitin sulphates (CSs), especially those derived from marine sources, may have therapeutic benefits for patients with osteoarthritis, including those with a history of a systemic inflammatory response [9]. Chondroitin sulphates not only help to maintain the structural integrity and lubrication of joint tissues but also possess several other beneficial properties, such as bio-regulatory, anti-inflammatory, and anti-nociceptive effects.
Overall, the beneficial properties of CSs make them a promising treatment option not only for joint disorders such as osteoarthritis but also for comorbid pathologies, including those associated with traumatic injury. The aim of this review is to provide a comprehensive overview of the current literature on the molecular mechanisms underlying the more severe course of PTOA.
Additionally, we will evaluate the therapeutic potential of CS formulations with the best bioavailability and pharmacological properties in the management of PTOA and comorbid pathologies associated with the systemic inflammatory response. We hope that this review will provide valuable insights into the pathophysiology of PTOA and post-traumatic conditions characterized by a systemic inflammatory response, and contribute to the development of more effective treatments for patients suffering from this debilitating condition.
The sources used to write the literature review on the problem mentioned above include peer-reviewed scientific articles from reputable journals, systematic reviews and meta-analyses, clinical trials, and observational studies. Additionally, we have consulted textbooks and other reference materials in the fields of rheumatology, orthopaedics, traumatology, molecular medicine, and pathophysiology. The sources were identified through comprehensive searches of electronic databases such as PubMed, Scopus, Web of Science, and Cochrane Library, as well as manual searches of relevant reference lists.
Pathogenic mechanisms underlying post-traumatic osteoarthritis
The mechanism responsible for PTOA involves multiple pathways leading to disruption of cartilage matrix integrity, synovial inflammation, and subchondral bone alterations. After an articular fracture, including the release of blood and marrow contents into the joint space with the potential for systemic polytrauma, three mechanisms for the development of PTOA have been proposed (Table I).
Table I
Pathogenic mechanisms associated with the pathogenesis of post-traumatic osteoarthritis
| Pathogenetic mechanisms | Links in the pathogenesis | References |
|---|---|---|
| Disruption of cartilage matrix integrity | Mitochondrial dysfunction in chondrocytes | [5, 10] |
| Oxidative and nitrosative stress in articular cartilage | [11–13] | |
| Inflammatory response in articular cartilage | [14, 15] | |
| Chondrocyte programmed (apoptosis, pyroptosis, necroptosis, and ferroptosis) and non-programmed (necrosis) death | [16, 17] | |
| Hypercatabolism and diminished anabolism in articular cartilage | [18, 19] | |
| Altered joint biomechanics | [20, 21] | |
| Synovial inflammation | Mitochondrial dysfunction in synoviocytes | [5, 22] |
| Oxidative and nitrosative stress in synovium | [12] | |
| Inflammatory response in synovium mediating synovial macrophage and fibroblasts | [5, 23] | |
| Macrophage infiltration of synovium | [24] | |
| Subchondral bone alterations | Uncoupled bone remodelling and formation | [5, 25] |
One mechanism for the development of PTOA involves chondrocytes undergoing various forms of cell death, including programmed (apoptosis, pyroptosis, necroptosis, and ferroptosis) and non-programmed (necrosis), as a result of direct mechanical injury and joint structure incongruity, which leads to an insufficient number of viable chondrocytes to maintain the complex cartilage matrix.
The second mechanism involves an acute inflammatory response in the synovium that can potentially develop into a chronic process resulting in degenerative alterations within the joint. The third mechanism is associated with uncoupled bone remodelling characterized by reduced bone formation, peritrabecular fibrosis, and a significant increase in the breakdown of bone tissue. This can cause subchondral bone alterations [5].
The clinical symptoms of PTOA probably result from a combination of mechanisms that are influenced by various factors at the molecular, cellular, joint, and systemic levels. By improving our understanding of these pathological processes, we may be able to develop novel treatments that can prevent joint deterioration [5, 6].
Inflammatory response in articular cartilage and synovium in post-traumatic osteoarthritis pathogenesis: role of extracellular matrix molecules and redox-sensitive transcription factors
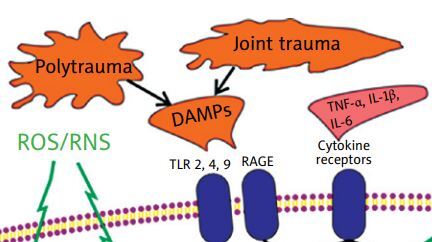
The inflammatory reaction that occurs as a consequence of joint fracture is possibly a noteworthy factor that contributes to the progression of PTOA, although its impact is not entirely understood [5, 6]. This damage can trigger low-grade systemic inflammation, which is characterized by an increase in cytokine release, leukocyte infiltration, and activation of pro-inflammatory transcription factors such as NF-κB.
In the case of PTOA, a significant role in initiating and sustaining the inflammatory response in the articular cartilage and synovium is attributed to damage-associated molecular patterns (DAMPs), which are endogenous molecules that are released from damaged cellular organelles (e.g., mitochondria) or dying cells (e.g., high-mobility group box protein 1 [HMGB1], calcium-binding protein S-100, uric acid) as well as an injured extracellular matrix (such as glycoproteins, proteoglycans, or glycosaminoglycans), and enter the joint cavity during cartilage breakdown [26, 27].
When these DAMPs are released into the synovial cavity, they prompt the production and release of inflammatory mediators by synovial cells such as macrophages and fibroblasts into the synovial fluid. The DAMPs exert their biological effects by binding to specific pattern recognition receptors (PRRs), which include Toll-like receptors (TLRs), NOD-like receptors (NLRs), and receptors for advanced glycosylation end products (RAGEs) [27, 28].
Pattern recognition receptors have been found on the surface of various cells, including immune cells, chondrocytes, osteoblasts, and synoviocytes, and their binding with DAMPs activates downstream signalling cascades, ultimately leading to the activation of transcription factors such as NF-κB [19, 29, 30]. Moreover, NF-κB becomes activated in osteoarthritic chondrocytes under excessive loading regimes [31].
This activation prompts the release of pro-inflammatory cytokines (e.g., tumour necrosis factor α [TNF]-α, interleukin 1β [IL-1β] and IL-6), chemokines (e.g., C-C motif chemokine ligand [CCL]-2, -5, -7, and -8), cathepsins (B, K, and L), the complement cascade as well as catabolic factors such as matrix metalloproteinases (MMP-1, -3, -9, and -13) [27, 28].
Matrix metalloproteinases and/or aggrecanases, specifically a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTS)-4 and -5, have the ability to break down numerous extracellular matrix molecules (e.g., fibronectin fragments, hyaluronan, tenascin-C, lubricin, fibromodulin, osteoadherin, chondroadherin, biglycan, native type II and IX collagen, N-terminal telopeptide of collagen type II, 24-mer synthetic peptide of type II collagen, aggrecan 32-mer fragment) [27, 28], which promote an NF-κB-dependent systemic inflammatory response and cartilage loss [32].
Moreover, NF-κB activation promotes the production of reactive oxygen and nitrogen species (ROS/RNS), which contribute to oxidative/nitrosative stress. This can be corrected by specific NF-κB inhibitors [33, 34] and polyphenols [35–38].
Furthermore, ROS/RNS can act as secondary signalling molecules, resulting in increased activation of redox-sensitive pathways associated with MAPKs, including the p38 cascade, extracellular signal-regulated kinase 1/2 (ERK 1/2), and c-Jun N-terminal kinases (JNK), as well as STAT3, which play a crucial role in inducing oxidative and nitrosative stress and the production of pro-inflammatory cytokines [13, 39, 40], as well as in the degradation of cartilage [28, 41].
All of the aforementioned factors trigger a vicious cycle between the cartilage and synovial membrane, and are considered vital in the development of PTOA (Fig. 1). This process may be limited by the activation of a redox-sensitive transcription factor that is functionally antagonistic to NF-κB, known as nuclear factor erythroid-2-related factor 2 (Nrf2) [42, 43]. Nuclear factor erythroid-2-related factor 2 activation has the potential to suppress M1 polarization and encourage M2 polarization through various signalling pathways including TLR/NF-κB, JAK/STAT, and MAPK signalling [44].
Fig. 1
The role of damage-associated molecular patterns and redox-sensitive signalling pathways in the pathogenesis of post-traumatic osteoarthritis.
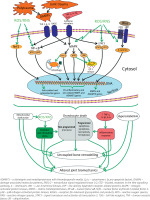
The canonical Wnt/β-catenin signalling is an another pathway up-regulated in joint trauma [45]. The maintenance of chondrocyte metabolic balance depends on baseline Wnt activity, while enhanced Wnt activity, as found in PTOA, can cause related extracellular matrix-degrading proteases to degrade cartilage matrix [46].
Matrix metalloproteinase-13, ADAMTS-4 and -5 expression levels were significantly increased in β-catenin overexpressing animals [47]. In mice, inhibition of the canonical Wnt pathway was found to reduce the PTOA severity by promoting anticatabolic effects on chondrocytes and antifibrotic effects on synovial fibroblasts [48].
The development of joint trauma and/or polytrauma is accompanied by the release of intracellular and extracellular DAMPs, which interact with specific pattern recognition receptors, such as TLRs and RAGEs. As a result, signalling pathways associated with NF-κB and MAPKs, including ERK1/2, p38, and JNK, are activated. This leads to the expression of pro-oxidant (Cyp7b, Cyp2C11, Cyp2E1, gp91 phox, inducible NO-synthase, etc.) and pro-inflammatory genes (TNF-α, IL-1β, IL-6, chemokines, cathepsins, phospholipase A2, cyclooxygenase 2, etc.).
As a consequence, there is an increase in the production of ROS/RNS, which are capable of maintaining long-term activity of the signalling pathways associated with NF-κB, MAPKs, STAT3, Wnt/β-catenin, even in the absence of action from other receptor stimuli. On the other hand, pro-inflammatory cytokines also increase the activation of NF-κB, MAPKs, and STAT3.
All of this, in turn, enhances the further increase in the expression of pro-oxidant, pro-inflammatory, MMPs, and ADAMTS genes. Moreover, the activation of certain MAPKs promotes mitophagy and the release of cytochrome c from mitochondria, which are accompanied by mitochondrial dysfunction and initiation of apoptosis.
All of these changes lead to programmed and non-programmed (necrosis) death of chondrocytes, the development of oxidative and nitrosative stress, the inflammatory response, hypercatabolism, resulting in the main manifestations of PTOA, such as disruption of cartilage matrix integrity, synovial inflammation, and subchondral bone alterations.
The redox-sensitive Nrf2-associated pathway, which induces the expression of antioxidant and cytoprotective genes, is functionally antagonistic to the signalling systems described above. Thus, targeting signalling pathways that are associated with redox-sensitive transcription factors could be a potential therapeutic strategy for preventing or treating PTOA [19, 27].
Chondroitin sulphates in the pathogenetic therapy of systemic inflammatory response: perspectives and risks
Chondroitin sulphates are a class of glycosaminoglycans that play a critical role in the structure and function of various tissues, including cartilage, bone, and connective tissue. They are composed of a chain of repeating disaccharide units, with each unit consisting of a hexosamine (either N-acetylgalactosamine or N-acetylglucosamine) and a uronic acid (either glucuronic acid or iduronic acid).
Chondroitin sulphates, in the form of chondroitin-4-sulphate and chondroitin-6-sulphate, are the most common glycosaminoglycans in the human body and are important components of aggrecan – the main proteoglycan of the cartilage matrix. They participate in the regulation of cell adhesion, proliferation and differentiation, transport of water, amino acids and lipids in hyaline cartilage, and determine the key biomechanical properties of the cartilage tissue (its elasticity), as well as the viscosity of the synovial fluid [49].
Numerous clinical studies and meta-analyses have provided evidence for the recommendation by the European League Against Rheumatism (EULAR, now – European Alliance of Associations for Rheumatology) and other influential medical organizations to use CS-containing agents as symptomatic slow-acting drugs for osteoarthritis (SYSADOA) for the treatment of osteoarthritis [50].
Recent meta-analyses have also demonstrated significant positive effects of CSs on the structure of cartilage and bone tissue, as well as joint function in osteoarthritis, with moderate reduction of chronic pain syndrome [51, 52].
In the context of systemic inflammation, CSs have been investigated as potential therapeutic agents due to their anti-inflammatory and anti-oxidant properties. Studies have suggested that CSs can inhibit the activity of inflammatory cytokines and enzymes, and modulate the expression of adhesion molecules on the surface of immune cells [53].
Chondroitin sulphates restricted lipid peroxidation, restored the natural antioxidants such as reduced glutathione and superoxide dismutase, lowered the levels of plasma TNF-α, and limited the infiltration of synovial neutrophils in mice with collagen-induced arthritis [54].
In the same model, it was found that CSs can reduce the levels of the pro-inflammatory IL-6 [55]. Additionally, it was observed that dietary supplementation with CSs could reduce IL-1β in joint tissue in Freund’s adjuvant arthritis [56].
Finally, several in vitro studies support the view that CSs can reduce inflammation by acting on the nuclear translocation of NF-κB, which is closely related to such indicators of the systemic inflammatory response as serum concentrations of IL-1, IL-6, and C-reactive protein [57, 58]. These statements were confirmed when using a model of LPS-treated mouse articular chondrocyte cultures [59], as well as by reproducing an experimental collagen-induced arthritis in mice [60].
It has been demonstrated that CSs not only suppress NF-κB activation, but also impact another pathway involved in the production of pro-inflammatory proteins, which is associated with the activation of ERK1/2 and p38 MAPK [61–64]. In addition, CSs inhibit the movement of STAT3 into the nucleus and markedly decrease the transcriptional activity of STAT3 through a mechanism that is not related to STAT3 phosphorylation [65].
All of this led to a decrease in the production of not only cytokines (in particular, IL-1β and TNF-α), but also a number of pro-inflammatory enzymes (phospholipase A2, cyclooxygenase 2, inducible isoform of NO synthase). It has also been shown that exogenous chondroitin sulphates can inhibit Wnt3a signalling in fibroblasts [66].
On the other hand, CSs demonstrate anti-inflammatory and antioxidant effects by up-regulating Nrf2 [67], which is functionally antagonistic to NF-κB. The mechanism of CS action provides their anti-inflammatory, anti-oxidant, and anticatabolic effects, which are beneficial for the morphological and functional state of cartilage, synovial membrane, and subchondral bone.
Chondroitin sulphates prevent the development of systemic inflammation, which is important to consider in the simultaneous pathogenetic treatment of osteoarthritis and comorbid conditions accompanied by a cytokine storm of varying intensity, including those associated with polytrauma and COVID-19. Moreover, CSs exhibit antiviral and anti-infective effects and may be beneficial for tissue engineering [68].
Experimental and clinical evidence suggests that CSs can be useful therapeutic agents for inflammatory bowel disease, atherosclerosis, periodontitis, Parkinson’s and Alzheimer’s diseases, multiple sclerosis, amyotrophic lateral sclerosis, psoriasis, rheumatoid arthritis, and systemic lupus erythematosus [62, 69, 70].
In addition, CSs have been reported to exhibit anabolic/anticatabolic and anti-apoptotic properties both in vitro and in vivo [53]. Chondroitin sulphates have been found to have several beneficial effects, such as increasing proteoglycan production in rabbits with cartilage degradation [71], preventing the increase of MMP-9 in rats with Freund’s adjuvant arthritis when given as a dietary supplement [56], and inhibiting MMP-13 in mice with collagen-induced arthritis [60].
Interestingly, the inhibition of MMP-13 may occur through the inhibition of p38 and ERK1/2 activation [72]. Chondroitin sulphates have also been shown to inhibit MMP-1, -3, and -13, as well as ADAMTS-4 and -5 in human, bovine, and porcine chondrocytes [53].
The anti-apoptotic effect of CSs, including their ability to decrease the sensitivity of rabbit chondrocytes to apoptosis, is also linked to the inhibition of NF-κB translocation and the MAPK signalling pathway via p38 and ERK1/2 [61].
Some meta-analyses indicate some contradictions in the results of clinical trials, which are associated with the risks of bias, brand, and sample size [51, 52]. Sometimes the use of CSs did not improve pain or functional status of joints in osteoarthritis [73].
Such ambiguous results of clinical trials may be related to the prescription of CSs to patients in different dosage forms (for oral or parenteral use), or made from different raw materials and in the presence or absence of certain physiologically active compounds (other glycosaminoglycans, amino acids, minerals, etc.).
For example, CSs derived from bovine and porcine raw materials are characterized by a high content of sulphate groups in the 4th position and low charge density, which are responsible for the retention of CSs on hyaluronic acid.
In addition, the animal origin of these products poses a potential hazard to consumers due to possible contamination of raw materials with prions, which cause bovine spongiform encephalopathy and are etiologic factors of Creutzfeldt-Jakob disease in humans, or due to restrictions on use related to religious beliefs [74].
Synthetically derived CSs are characterized by the presence of tri- and tetra-sulphate groups that are not found in naturally occurring CSs, which is associated with the low biocompatibility of such drugs. At the same time, CSs derived from marine organisms have a low content of sulphate groups in the 4th position, and high density of anionic charge, which contributes to the retention of CSs on hyaluronic acid [74–77].
Chondroitin sulphate <GlcA-GalNAc(4S,6S)> chains isolated from marine organisms exhibit antiviral and antimetastatic activity, have certain signalling properties, and improve the mechanical characteristics of cartilage tissue [75]. Chondroitin-6-sulphate obtained from sharks can counteract IL-1β inhibition of tissue inhibitor of metalloproteases (TIMP)-3 in human chondrocytes and TIMP-1 in synovial fibroblasts, while chondroitin-4-sulphate derived from porcine trachea did not have the same effect [76, 77].
Vassallo et al. [78] conducted a study to examine how primary pathological synoviocytes responded to different animal-derived chondroitins, namely bovine, pig, fish, as well as unsulphated biofermentative chondroitin. All samples displayed expected anti-inflammatory properties; however, bovine and fish CSs and biofermentative chondroitin affected more cytokines and chemokines.
In Western blot analyses, fish CSs and biofermentative chondroitin were comparable in their ability to down-regulate osteoarthritis-related biomarkers such as NF-κB, mechanistic target of rapamycin (mTOR), pentraxin 3, and cartilage oligomeric matrix protein. Proteomic analyses revealed the modulation of both common and distinct molecules to chondroitin treatments.
Thus, fish CSs and biofermentative chondroitin demonstrated the ability to modify biological mediators involved in the inflammation cascade, matrix degradation/remodelling, glycosaminoglycan synthesis, and cellular homeostasis.
López-Senra et al. [79] conducted a study to evaluate the effect of CSs derived from different sources, including small spotted catshark (Scyliorhinus canicula), blue shark (Prionace glauca), thornback skate (Raja clavata), and bovine CSs (reference), on the proliferation of osteochondral cell lines (MG-63 and T/C-28a2) and the chondrogenic differentiation of mesenchymal stromal cells (MSCs).
They reported that intermediate values of chondroitin-4-sulphate and chondroitin-6-sulphate enhanced cell proliferation and have the potential to promote chondrogenesis. Regarding the specific cell lines, MG-63 proliferation was similar between R. clavata (with intermediate chondroitin-6-sulphate ratio) and bovine CSs (with chondroitin-6-sulphate enrichment).
Osteochondral cell line T/C-28a2 proliferation was significantly improved by intermediate ratios of chondroitin-4-sulphate and chondroitin-6-sulphate, particularly those derived from S. canicula and R. clavata. A dose-dependent response was observed for S. canicula and bovine CS. The sulphation patterns of CSs also had a discrete effect on MSC chondrogenesis.
Several preclinical studies have demonstrated the potential of bioactive extracts of fish in the treatment of PTOA. In one study, oral fish cartilage hydrolysate accelerated joint function recovery in a rat model of traumatic knee osteoarthritis [80].
Another study showed that the oral administration of an extract from the snakehead fish (Channa striatus) to rabbits with experimentally induced PTOA in a stifle joint, following transection of the anterior cruciate ligament, led to a significant reduction in soft tissue swelling observed in radiographs of the animals 9 weeks after treatment. Additionally, an improvement in the density of nerve fibres in the synovial membrane of rabbits was observed [81].
However, the use of CSs in the pathogenetic therapy of systemic inflammation also poses potential risks. These include the risk of adverse reactions, such as gastrointestinal upset, as well as the risk of drug interactions with other medications.
Additionally, the quality and purity of commercially available CS preparations may vary, which can impact their efficacy and safety. Some of the CSs have undesirable effects associated with the risk of thrombosis, which is typical, for example, for the sodium salts of CSs A and C, which are contraindicated for use in thrombophlebitis [9].
Thus, given the peculiarities of the pathogenesis of the systemic inflammatory response and its complications in the SYSADOA, preference should be given to parenteral drugs that do not enhance the prothrombogenic properties of blood.
Overall, while CSs hold promise as a potential therapeutic agent for systemic inflammation, further research is needed to fully understand their mechanism of action and assess their safety and efficacy in clinical settings.
Purified bioactive extracts of small sea fish: a promising therapy for post-traumatic osteoarthritis
Chondroitin sulphates are present in high concentrations in bioactive extracts derived from certain fish species. In vitro studies have demonstrated that CSs obtained from fish possess a broad spectrum of pharmacological activities, including antioxidant effects (derived from tilapia viscera as well as salmon, shark, and ray cartilage), anticoagulant, anti-platelet, and thrombolytic effects (derived from Nile tilapia and pacu viscera, sturgeon skull, and backbone), anticancer effects (derived from grey triggerfish and smooth-hound skins), anti-obesity effects (derived from skate), anti-inflammatory and anti-dyslipidaemic effects (derived from skate cartilage), wound healing effects (derived from sturgeon cartilage and backbone), neuroprotective effects (derived from shark cartilage), and chondroprotective effects (derived from small spotted catshark, blue shark, thornback skate, and small sea fish) [68, 78–82].
Standardized small fish extract (SSFE) has been shown to have anti-inflammatory effects and stimulate the synthesis of new cartilage, which is associated with the composition and manufacturing process of the medications.
For example, a Romanian pharmaceutical compound of marine fish extract consists of 0.01 g of standardized purified bioactive extract of 4 species of small marine fish: Black Sea sprats (Sprattus sprattus phalericus), Black Sea merlin (Merlangius euxinus), Black Sea pufferfish (Alosa tanaica nordmanni) and Black Sea anchovy (Engraulis encrassicholus ponticus).
This extract, along with CSs (chondroitin-4-sulphate and chondroitin-6-sulphate), also contains other glycosaminoglycans (hyaluronic acid, dermatan sulphate, keratan sulphate), as well as low molecular weight polypeptides (molecular weight up to 50 kDa), amino acids (alanine, valine, leucine, isoleucine, arginine, proline, serine, threonine, asparagine, methionine, hydroxyproline, glutamic acid, phenylalanine, lysine, tyrosine) and minerals (sodium, potassium, iron, calcium, magnesium, copper, manganese, zinc) that play an important role in connective tissue metabolism [9].
The drug is intended for parenteral (intramuscular and intra-articular) administration to patients with primary and secondary osteoarthritis of various localizations, as well as degenerative-dystrophic diseases of the spine and soft tissue pathology.
In clinical trials, SSFE demonstrated chondroprotective (slowing the progression of osteoarthritis and the growth of osteophytes, reducing the degradation of the articular cartilage matrix), anti-inflammatory, anti-oxidant, and analgesic effects, and showed significant correction of joint and spinal function and improvement in quality of life [83–86].
The drug reduced the time of epithelialisation of ulcerative and erosive defects that occurred when taking NSAIDs, which allowed researchers to recommend it as a drug of choice in patients with osteoarthritis with NSAID gastropathy [87].
The use of SSFE as part of the initial treatment of musculoskeletal diseases in conjunction with NSAIDs accelerated the onset of the analgesic effect and reduced the need for NSAIDs [83].
The efficacy of SSFE in the complex pharmacotherapy of back pain has been shown, which is associated with its ability to attenuate afferent nociceptive activity by reducing inflammation in the spinal structures and decreasing central sensitization [88, 89]. The positive effect of the drug is its long-term (up to 1 month after the end of treatment) analgesic effect.
The patterns of SSFE effect on inflammation and regeneration have been elucidated in detail in a number of experimental studies that revealed its ability to suppress gene expression and release of proinflammatory cytokines (IL-1β, IL-6, IL-8), and inhibit the activity of aggressive proteolytic enzymes such as matrix metalloproteinases and ADAMTS4, resulting in reduced chondrocyte and osteocyte alteration and apoptosis [90–93]. An important mechanism of transcriptional inhibition of proinflammatory cytokines is the reduction of NF-κB expression, in particular, its p50 subunit (NF-κB1) [93].
Under the conditions of IL-1β and TNF-α stimulation, small marine fish extract effectively counteracted the decrease in the number of chondrocytes both by inhibiting apoptosis and stimulating their proliferation rate [94].
The regenerative effect of SSFE is confirmed by its ability to stimulate both chondrocyte proliferation (DNA synthesis and mitotic activity) and the synthesis of extracellular matrix components, such as aggrecan and hyaluronan [91, 93, 95]. In addition, the use of a standardized small fish extract also had a positive effect on the level of transforming growth factor β (TGF-β), a balanced pool of which is important for cellular regeneration of cartilage tissue [91].
The pharmacological effects of SSFE and its safety profile are particularly important for PTOA pharmacotherapy in patients with comorbidities associated with the development of a systemic inflammatory response of varying intensity. Firstly, this drug not only has the ability to reduce the release of pro-inflammatory cytokines but also to suppress their gene expression, including NF-κB-mediated [90–93].
This is important for the prevention and limitation ofpro-inflammatory hypercytokinaemia, including in polytrauma and COVID-19 and their complications, which maintain chronic inflammation – a leading pathogenetic mechanism of PTOA and many comorbid conditions.
Secondly, an important advantage of SSFE is its absence of negative impact on haemocoagulation parameters and reduced need for NSAIDs, which can mask early symptoms of diseases accompanied by a systemic inflammatory response. This distinguishes SSFE from other CSs, in particular, sodium salts of CSs A and C [9].
Another interesting point that justifies the advantage of SSFE over other members of the SYSADOA in patients with PTOA under the risk of developing a systemic inflammatory response, including in conditions of polytrauma and infectious diseases, is its low immunogenicity.
Meanwhile, products containing glycosaminoglycan-peptide complexes of bone marrow and intercostal cartilage of cattle have the potential to cause the formation of antibodies to themselves. Before starting treatment with such drugs, the patient is required to consult a doctor to exclude the presence of systemic autoimmune pathology (rheumatoid arthritis, ankylosing spondylitis, systemic lupus erythematosus, scleroderma). All these diseases are known to be accompanied by the development of a systemic inflammatory response [68].
In our opinion, the aforementioned benefits of SSFE-based preparations (such as a pharmaceutical compound of marine fish extract) may be attributed not only to their production technology (degreasing and deproteinization), but also to the presence of other physiologically active compounds (amino acids and minerals) within this complex preparation, in conjunction with CSs. It is known that cartilage injury leads to an increase in amino acid metabolism, including the biosynthesis of arginine and the metabolism of serine, threonine, and proline, among others [96].
For instance, the enzyme arginase catalyses the breakdown of arginine into ornithine, which is subsequently metabolized into proline, polyamines, and glutamine. Patients with treatment-resistant knee osteoarthritis exhibit a significant deficiency of Larginine [97]. It is also worth noting that L-arginine has the potential to alleviate lipopolysaccharide-induced inflammation by inhibiting the TLR4/NF-κB and MAPK pathways [98].
Another important component that sets the pharmaceutical compound of marine fish extract apart from other SYSADOA is its inclusion of minerals necessary for tissue repair. For instance, magnesium has properties that combat inflammation and oxidation, leading to the development of cartilage matrix and the improvement of chondrocyte reproduction, which effectively works against osteoarthritis. Zinc, on the other hand, is essential for antioxidant defence and suppressing prostaglandin synthesis. It also stimulates cartilage growth and development, and it further aids in differentiating mesenchymal stem cells into chondrocytes in osteochondral defects [99].
Conclusions
1. The mechanism responsible for PTOA involves multiple pathways that lead to the disruption of cartilage matrix integrity, synovial inflammation, and alterations in subchondral bone, all of which are molecularly linked to the activation of pro-inflammatory, pro-oxidant, and pro-histolytic transcription factors such as NF-κB, MAPKs, and STAT3.
The activation of these factors leads to a systemic inflammatory response, oxidative and nitrosative stress, mitochondrial dysfunction in chondrocytes and synoviocytes, programmed and non-programmed death of chondrocytes, hypercatabolism, and diminished anabolism in articular cartilage, altered joint biomechanics, as well as uncoupled bone remodelling and formation.
2. Experimental and clinical studies have shown that CSs have the ability to improve the structure and function of cartilage and subchondral bone, which is associated with their ability to decrease the activation of NF-κB and p38 MAPK, and up-regulate Nrf2. These properties of CSs are associated with their anti-inflammatory, antioxidant, anabolic/anticatabolic, and anti-apoptotic properties.
3. Pharmacological effects of CSs are greatly dependent on the pharmaceutical form (oral or parenteral), the type of raw material used for their preparation, and the presence of specific physiologically active compounds (such as other glycosaminoglycans, amino acids, and minerals).
4. Considering the pathogenesis of PTOA, it is advisable to use parenteral preparations of CSs that do not increase the pro-inflammatory and pro-thrombogenic properties of blood. Standardized small fish extract is an example of the drugs that can attenuate NF-κB-mediated systemic inflammation, potentially helping to reduce joint inflammation and cartilage degradation, improve joint function, and alleviate pain and disability in patients with these conditions.
Standardized small fish extract is one among the drugs that have the potential to attenuate NF-κB-mediated systemic inflammation, which may help in diminishing joint inflammation and cartilage degradation, enhancing joint function, and relieving pain and disability in patients affected by these conditions.
5. The presence of certain amino acids (such as arginine, serine, threonine, and proline) and minerals (such as magnesium and zinc) in the composition of SSFE-based preparations may also enhance the effectiveness of PTOA treatment.