Introduction
Multiple sclerosis (MS) is an autoimmune disorder affecting the central nervous system (CNS), including both the brain and spinal cord. It is characterized by inflammatory and demyelinating changes within the CNS. It is considered a result of an inflammatory disease of still unclear etiology and chronic course with demyelinating and inflammatory changes in the CNS.
The CNS lesions are multifocal and multitemporal, and the main method in the initial diagnostic period is magnetic resonance imaging (MRI), which is undoubtedly the best tool for monitoring the course of the disease [1–3]. Moreover, an MRI is essential for monitoring the disease’s course. The disease involves the immune system mistakenly attacking the myelin sheath that surrounds nerve fibers, leading to disruptions in the transmission of nerve signals.
Multiple sclerosis is more prevalent in women, with a ratio of 2–3 times higher than in men. The typical age of onset falls between 20 and 50 years, although, in around 0.5% of cases, it can start at age 60 or older [4, 5].
The treatment of MS, as in rheumatic diseases (RD), different approaches are used, depending on the form of the disease, its severity and the patient’s symptoms. For both MS and some RD, an individualized approach to treatment, taking into account both clinical symptoms and the patient’s response to therapy, is crucial. Therapies may include disease-modifying therapies (DMTs), which reduce disease activity and the number and severity of exacerbations. There are also symptomatic therapies such as painkillers, exercise rehabilitation and supportive therapies.
In rheumatology, the treatment of RD also includes different approaches. Anti-inflammatory drugs, disease-modifying antirheumatic drugs (DMARDs), glucocorticosteroids, as well as physical and rehabilitation therapies are used. There is also controversy regarding the use of various biological drugs, among others tumor necrosis factor inhibitors (TNFi) in the context of risk of demyelination during such treatment or discussion about risk of exacerbation of diagnosed previously MS. However correlation between TNF plasma levels in patients, could reflect the activity of pathological processes in both RD and MS.
There are to main types of MS. Relapsing-remitting (RR) is the most common course of MS. It is characterized by episodes of neurological deterioration, known as flares or relapses, followed by periods of remission where symptoms improve or stabilize. The disease follows a pattern of fluctuations between these relapses and remission.
Secondary progressive (SP) is the MS form which typically follows a RR course initially. However, over time, the disease changes to a progressive course where there is a gradual worsening of symptoms and disability, with fewer or no distinct relapses. This shift from RR to SP is a notable feature of this form.
Primary progressive (PP) type is the rarest form of MS, characterized by a gradual progression of neurological symptoms from the beginning of disease development. These different forms of MS reflect the varying patterns of disease progression and symptom presentation. Relapsing-remitting MS is marked by its episodic nature, SP MS involves a transition from relapses to continuous progression, and PP MS represents a steady and unrelenting progression of symptoms [1, 3, 5].
Multiple sclerosis stands as the leading cause of non-traumatic disability in young adults. Neurological symptoms affecting the musculoskeletal system in MS patients encompass muscle weakness, spasticity, heightened reflexes in tendons, and the occurrence of abnormal clinical signs like Babinski’s sign.
The implementation of immunomodulatory medications has yielded notable effects in decreasing the frequency of MS episodes, impeding the development of fresh demyelinating lesions within the CNS, and decelerating the progression of disability. This has been a significant advancement in the management of the disease [3, 6].
According to information obtained from the Polish National Health Fund, there were 2,400 new cases of MS reported in 2016, corresponding to a rate of 6.3 new patients for every 100,000 individuals in the population. The total number of registered cases of MS in 2016 was 42,400 patients, resulting in an incidence rate of 110 cases per 100,000 people in the Polish population. It’s estimated that approximately 45,000 individuals in Poland are affected by MS. On a larger scale, across Europe, there are roughly 700,000 individuals living with MS. Globally, the number of individuals with MS exceeds 2.5 million [4].
The improved accessibility of MS patients to course-modifying treatment in Poland with its fairly well-developed network of MS drug program providers suggests simplifying enrollment in drug programs, thereby shortening the time to implement specialized treatment [7]. Treatment of patients with MS includes a variety of health services in hospital treatment in drug programs, outpatient specialized care, and medical rehabilitation, where programs are created to improve MS patients with the participation of the Polish Rehabilitation Society and cooperation with the National Health Fund. Up to now, there is no clear explanation of the availability of the MS pharmacological treatment in Poland and it can be assumed that the same reason is common in most of the developed countries in the United Europe.
To date, the reasons behind the availability of pharmacological treatment for MS patients in Poland remain unclear. It is reasonable to assume that similar factors may contribute to this issue in many other developed countries within the European Union [8].
The objective of this observational study is to shed light on the factors that contribute to delays in the initiation of pharmacological treatment for MS patients. This will be accomplished through an examination of the management practices within the Greater Poland region. The study seeks to offer insights into why treatment initiation may be delayed, thus potentially providing valuable information for improving the process and enhancing the overall care provided to MS patients. A review of the available literature indicates a lack of descriptions of the analysis of this phenomenon in Poland.
Subjects and results
Based on the data gathered from an internal study on the treatment of MS in the Greater Poland region, which was analyzed during the period of 2017 to 2018 (from January to October), the study focused on the implementation of drug programs within several healthcare facilities. The participating institutions included facilities in Poland: Independent Public Health Care Center of the Ministry of Internal Affairs and Administration in Poznan, Heliodor Święcicki Clinical Hospital in Poznan, Multispecialty City Hospital with Nursing and Medical Care Department Independent Public Health Care Facility located in Poznan, Specialized Hospital in Piła, Voivodeship Integrated Hospital in Konin.
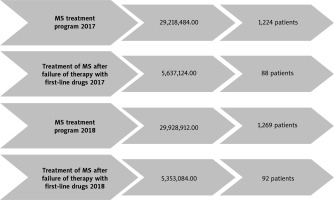
During the study period, a total of 1,224 MS patients were included in treatment programs in 2017, with the overall cost of the program amounting to PLN 29,218,484.00 (about 6,492,996.4 Euros). Following the failure of treatment with first-line drugs, 88 patients were identified and qualified for further treatment, which incurred a cost of PLN 5,637,124.00 (about 1,252,694.00 Euros)
In the subsequent 2018 year, the drug program enrolled a larger cohort of 1,269 MS patients, with a program value of PLN 29,928,912.00 (about 6,650,869.00 Euros). Among them, 92 patients were qualified for advanced treatment after experiencing the failure of first-line drug treatment, which carried a cost of PLN 5,353,084.00. These data provide a comprehensive overview of the patient enrollment, program costs, and qualifications for advanced treatment within the MS drug programs during the specified timeframe in the Greater Poland region (Fig. 1).
Fig. 1
Data on multiple sclerosis pharmacological treatment program in Greater Poland. General characteristic in January–October 2017 and 2018 years. Based on data from Polish National Health Fund with own observations. Abbreviation: PLN – polish zloty currency.
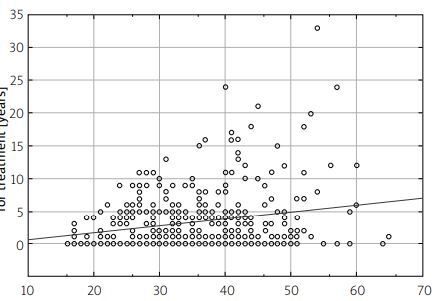
The analysis of factors affecting a patient’s waiting time to qualify for the program reveals that the average duration between the onset of initial symptoms and the diagnosis of MS was approximately 1.4 years. This finding indicates the period of time that typically elapses before a patient receives an official diagnosis of MS after the appearance of their initial symptoms (Fig. 2 A). In contrast, the average waiting time for a patient after receiving a diagnosis of MS to be referred for qualifying treatment was approximately 3.5 years, as shown in Figure 2 B.
Fig. 2 A
The patient waiting time for clinical evaluation and the final diagnosis, starting from the onset of initial symptoms to the confirmation of diagnosis, is an essential factor in the management of multiple sclerosis.
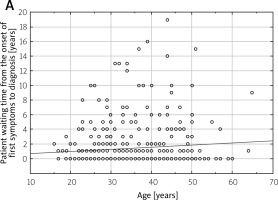
Fig. 2 B
The patient waiting time from the point of diagnosis of multiple sclerosis (MS) to the moment of being referred for qualification for treatment is a significant factor in the patient care pathway.
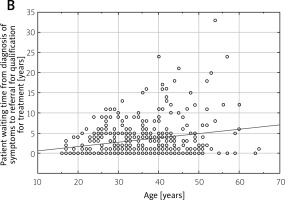
This waiting period signifies the duration that patients have to wait from the point of being diagnosed with MS to the time they are referred for eligibility assessment for treatment. It’s noteworthy that among all the patients who were diagnosed with MS, the largest group, accounting for 69.4%, fell into the active working age group. This statistic highlights that a significant portion of individuals affected by MS and requiring treatment are in their prime working years.
Reducing the waiting time for treatment qualification referrals can be crucial in ensuring that these patients receive timely access to the appropriate therapies, which can potentially impact their overall well-being, productivity, and quality of life.
Discussion
The current standard for diagnosing MS is the McDonald criteria, which were revised and published in 2017 [6]. These criteria build upon the earlier 2010 McDonald criteria and are endorsed by the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) and the US National Multiple Sclerosis Society. An expert group called the Magnetic Resonance Imaging in Multiple Sclerosis (MAGNIMS) has also proposed criteria that aim to establish an MS diagnosis efficiently. According to these guidelines, a diagnosis of MS involves confirming both dissemination in time (DIT) and dissemination in space (DIS).
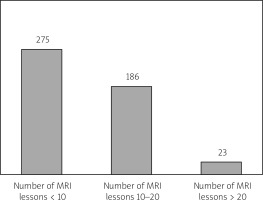
In other words, the criteria require evidence of multiple neurological events occurring at different times and locations. This approach aims to ensure that the diagnosis is accurate and based on clear clinical and imaging evidence. In eligible patients meeting specific MRI criteria, the largest subgroup consists of individuals with fewer than 10 lesions observed on MRI scans, primarily located in the brain. These MRI findings are used in conjunction with clinical assessments to support the diagnosis of MS. These guidelines represent a collaborative effort to establish a more efficient and accurate diagnostic process for MS, taking advantage of advancements in imaging technology and clinical understanding [5, 6, 9, 10] (Fig. 3).
Fig. 3
The number of MRI lesions at the time of inclusion in the MS treatment program serves as a significant indicator in assessing the disease’s severity and progression.
The treatment of MS is still based on preventing disease progression and achieving the longest possible remission. The introduction of immunomodulatory drugs, has had a significant impact on reducing the frequency of relapses and inhibiting the formation of demyelinating lesions in the CNS and slowing the progression of disability [5].
The first drug introduced in 1993 was interferon β-1b (IFN-β-1b), and further clinical trials allowed the introduction of further IFN-β-1a-drugs. Interferons β are a group of drugs with multidirectional mechanisms of action that are not fully understood. Originally, their mechanism of action was described as antiviral, anti-tumor, antimitotic [6, 11].
In studies conducted in the 1980s, they were also proven to exhibit a number of immunomodulatory actions, and are known to stimulate CD8+ suppressor lymphocytes and inhibit the activity of CD4+ helper lymphocytes, thereby reducing demyelination of inflammatory origin, and additionally inhibit the induction of Th17 lymphocytes. The effect on cytokine secretion (decreasing interleukin-12 [IL-12] and IFN-γ, and increasing IL-4 and IL-10) and expression of adhesion molecules (e.g., vascular cell adhesion molecule [VCAM]) have been found. This reduces the permeability of the blood-brain barrier, while limiting the migration of T cells into the CNS.
The treatment approach for MS continues to focus on two main goals: preventing the progression of the disease and achieving prolonged periods of remission. The introduction of immunomodulatory drugs has had a significant positive impact on achieving these goals by reducing the frequency of relapses, inhibiting the formation of demyelinating lesions in the CNS, and slowing the progression of disability [5, 12].
Currently, we have 13 reimbursable drugs available for use in patients with the RR form of MS under the program of Polish National Health Fund, and these include glatiramer acetate, peginterferon β-1a, dimethyl fumarate, teriflunomide, fingolimod, natalizumab, ocrelizumab, cladribine and alemtuzumab, methylprednisolone, cyclophosphamide, mitoxantrone, azathioprine, among others [8].
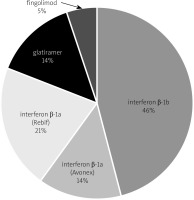
The increasing choice of drugs available for MS patients allows individualization of therapy and selection of the best formulation for the patient. Of the 500 patients treated at the Greater Poland center between 2011 and 2017, the largest group were women – 352, then men – 148. These patients were mainly treated with IFN-β-1b in 228 cases, IFN-β-1a – Rebif in 106 cases, glatiramer in 70 cases, IFN-β-1a – Avonex in 69 cases, fingolimod in 26 cases, dimethyl fumarate in 1 patient (Fig. 4).
This diversity in medication choices reflects the healthcare provider’s ability to tailor treatment plans to each patient’s unique requirements [8, 12–15].
The most well-known and widespread scale to score MS progression and monitor the effectiveness of medications used is currently the Expanded Disability Status Scale (EDSS) (Fig. 5). The 11-point scale DSS (Disability Status Scale) (from 0 – no disability to 10 – death due to MS) was first introduced in 1955 by American neurologist John F. Kurtzke [10, 16].
Fig. 5
The average classification score at the time of a patient’s qualification for the program is 5.9 on the Expanded Disability Status Scale (EDSS).
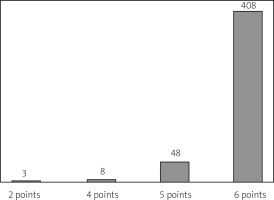
In 1983, the scale was expanded to a 20-item version of the EDSS, which lists scores between basic degrees of disability [16]. It consists of functional subclasses to assess visual, brainstem, pyramidal tract, cerebellum, sensory, sphincter and higher cerebral functions, and also includes an assessment of mobility and self-care abilities [16].
An additional tool used for assessing the condition of MS patients is the Ashworth scale. This scale gauges resistance during passive soft tissue stretching performed by the examiner. It employs a five-point scale, with 0 indicating no increased muscle tone and 4 signifying the absence of movement due to stiffness in flexion or extension. Complementing this, the Lovett scale assesses muscle strength across six grades, ranging from 0 (no visible voluntary muscle contraction) to 5 (normal muscle strength, allowing the patient to perform exercises with resistance). These scales collectively offer valuable methods for evaluating various aspects of a patient’s MS condition and monitoring changes over time [16].
The sensory perception test can use calibrated silicone von Frey monofilaments and include three tactile tests at dermatomal innervation sites (C4–S1). If the subject reports touch sensation twice, this is considered as a positive test result. Perception elicited by pressure with a 0.30 mm diameter fiber corresponds to normal perception, with a 0.12 mm diameter fiber corresponds to hypersensitivity, and with a 0.55 mm diameter fiber corresponds to hypersensitivity [17, 18].
Neurophysiological tests such as evoked potentials (visual evoked potentials – VEP , somatosensory evoked potentials – SSEP, brainstem auditory evoked potentials – BAEP) are very useful in detecting functional disorders of the nervous system including diagnostic of MS patients. Among neurophysiological tests, the most sensitive is up to 84% with confidently diagnosed MS are the motor evoked potentials (MEPs), which allow tracking recovery from relapses, as well as quantifying the extent of damage [19–22].
These neurophysiological assessments contribute to a comprehensive understanding of a patient’s neurological condition and assist healthcare professionals in accurately diagnosing and monitoring the progression of MS. Motor evoked potentials MEPs can reveal clinically silent the plaques of demyelination. It has been shown that 10–16% of MS patients without pyramidal symptoms showed subclinical changes in motor evoked potentials, indicating discrete damage to corticospinal tracts. Elevated excitability threshold and slowing or conduction block were demonstrated. However, MEPs testing is an underestimated method in Poland.
The current system of qualifying patients with MS for the treatment, despite significant improvements, is still limited compared to other European countries. Too late initiation of therapy with immunomodulatory drugs, as well as limitations in qualifying patients for treatment with second-line drugs (up to 60 months) and the lengthy process of allowing new drugs for reimbursement (3–7 years after drug registration), are just some of the problems affecting the gap between epidemiology and treatment application in MS patients (Fig. 6). The model of care should be based on neurological centers specializing in multidisciplinary care of MS patients.
Fig. 6
The average classification score at the time of patient’s qualification for the program – 5.9 on the EDSS scale.
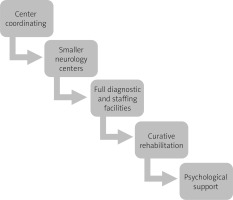
Such multidisciplinary teams should include, apart from neurologists, various specialists necessary in the process of MS diagnosis, differentiation and monitoring of the disease as well as potential comorbidities. Such team should undoubtedly include rheumatologists, especially in the case of overlap of MS and other autoimmune disease belonging to RD.
As well as observation and proper understanding of syndromes imitating MS in rheumatology may be the key to appropriate treatment. Some RD may present with neurological symptoms of MS. Thus, in the course of primary Sjögren’s syndrome and systemic lupus erythematosus (SLE) due to wide possibilities of nervous system involvement and disturbances of mental state are present with a range of neurological symptoms resembling MS [23–26].
Rheumatologists need to differentiate these symptoms to determine whether they are due to Sjögren’s syndrome or whether it is MS. Both diseases manifest common features such as symptoms resulting from damage to the brain, spinal cord and visual pathways, the detection of autoantibodies such as anti-nuclear, anti-Ro, anti-La antibodies and the presence of MRI abnormalities [24–26]. Almost all symptoms observed in people with CNS involvement in primary Sjogren’s syndrome can potentially be associated with concomitant MS.
The connection between RA and MS also was widely discussed. In some studies found presence of autoantibodies against myelin basic protein (MBP) in RA patients as well as a higher prevalence of RA in SM patients than in general population [27]. However results of different studies were conflicting. In the nationwide study by Tseng et al. [28] confirmed the increased incidence of RA in MS was revealed independently of RA traditional risk factors such as age, sex and smoking.
Sclerosis multiple-like syndromes can also be present in the course of ANCA associated vasculitis (AAV) [29]. Antineutrophil cytoplasmic antibodies (ANCA) can be present in MS and are associated with worse prognosis. Some authors suggested that genetic variants may predispose to AAV vasculitis and demyelinating disorders.
The real problem may create fibromyalgia (FM) with similar symptoms to MS such as: headaches, chronic fatigue, joint and muscle pain, numbness and tingling of extremities, memory problems (brain fog). In FM usually MRI brain or spinal lesions can’t be expected, the problem is when patient with FM and suspicion of MS has some MRI abnormalities [30].
Referring to elevated risk of demyelination during TNFi treatment, the role of TNF in MS was widely studied and analyzed to two TNF receptors: TNFR1 and TNFR2, which have opposite mode of action. Receptor TNFR1 mediates demyelination but TNFR2 has opposite abilities such remyelination and neuroprotection [31]. This knowledge may explain risk of demyelination using non-selective TNFi, while selective anti TNFR1 antibodies can be potential drugs in SM treatment, however it should be confirm in further studies.
In summary, the challenges presented in the treatment of patients with MS contribute to the gap between the epidemiological understanding of MS and the actual application of treatment. To address these issues, there is a call for a revised model of care. This model emphasizes the establishment of specialized neurological centers dedicated to providing comprehensive and multidisciplinary care for MS patients. Not only neurologists, but also physicians from other specializations, and in particular rheumatologists, need to be aware of these potential links between RD and MS and understand how certain rheumatological therapies may affect the nervous system, in particular the demyelinating processes in the CNS. This requires careful analysis, monitoring and therapeutic decision-making to minimize risks for patients with potential comorbidities.
This approach aims to bridge the gap between epidemiological insights and effective treatment by offering more streamlined and specialized care pathways. Such centers would likely provide more timely access to treatments, better monitoring, and improved coordination of care, ultimately enhancing the quality of life and outcomes for individuals with MS. The treatment of patients with MS should aim to enhance care by keeping up with advancements in knowledge and expanding diagnostic and therapeutic possibilities. Following clinical standards, the objective should be to extend the duration between diagnosis and the initiation of optimal therapy for MS patients.
Conclusions
Regarding MS therapy, patients should have access to drugs approved by clinical standards without restrictions imposed by payers on the duration of treatment. It is important to advocate for greater use of drugs classified as new therapies. It is important to advocate for a broader utilization of drugs categorized as new therapies. A recommended approach is to implement a coordinated care model for MS, integrating healthcare and social care activities centered around the patient and their family. This model should also embrace an interdisciplinary approach to patient care, acknowledging the significance of collaboration among various healthcare professionals.