Introduction
Rheumatoid arthritis (RA) is a chronic, systemic connective tissue disease of autoimmune origin and unknown etiology. Depending on the presence or absence of rheumatoid factors (RF) in the IgM class and/or antibodies against citrulline peptides, it is distinguished as either serologically positive or negative RA. Typically, RA is characterized by symmetrical arthritis, what with extraarticular and systemic symptoms may lead to disability, poor quality of life and premature death [1].
Rheumatoid arthritis affects 0.46% of global population. The highest pooled prevalence of RA is in North America – 0.70%, followed by Europe 0.54%. The lowest pooled prevalence is in South America – 0.30% and Asia – 0.30% [2].
The disease is more commonly diagnosed in women and can manifest at any age, with the highest incidence occurring in the fourth and fifth decades of life [1]. Genetic risk factors for RA include the presence of the human leukocyte antigen HLA-DRB1. Furthermore, the risk increases among tobacco smokers, overweight or obese individuals, those with low alcohol consumption, poor dental and low socioeconomic status [1].
In approximately 70% of cases, there are periods of exacerbations and relative remissions with progressive joint destruction. Nearly 10% of patients experience prolonged remissions lasting several years.
Typically, RA manifests as symmetrical pain and swelling of small joints in the hands and feet (proximal interphalangeal, metacarpophalangeal, and metatarsophalangeal joints). Involvement of larger joints such as knees, shoulders, elbows, and hips are also possible. General symptoms include low-grade fever, muscle pain, fatigue, loss of appetite, and weight loss. Extraarticular manifestations most commonly affect patients with serologically positive RA (RF and/or ACPAs positive) and are associated with a chronic course of the disease; these include rheumatoid nodules, cardiovascular, respiratory, ocular pathologies and renal involvement [3].
The multitude of complications and comorbidities accompanying RA build a complex medical picture of the patient, requiring an interdisciplinary approach during both the diagnostic and therapeutic processes.
Chronic kidney disease in rheumatoid arthritis
Chronic kidney disease (CKD) is defined as the presence of abnormal renal structure or function persisting for a minimum of 3 months, exerting an impact on health regardless of the underlying causative factors. Both, the severity of albuminuria (categorized as A1 to A3) and the glomerular filtration rate (GFR) stages (ranging from G1 to G5) serve as the fundamental basis for evaluating the progression of CKD [4].
Albuminuria categories are defined based on albumin excretion rate (AER) expressed in mg/day or albumin-creatinine ratio (ACR) expressed in mg/g: A1: < 30 mg/day or < 30 mg/g, A2: 30–300 mg/day or 30–300 mg/g, A3: > 300 mg/day or > 300 mg/g.
In the 5-stage GFR classification the following filtration rates apply: G1 – GFR ≥ 90 ml/min/1.73 m2, G2 – GFR 60–89 ml/min/1.73 m2, G3a – GFR 45–59 ml/min/1.73 m2, G3b – GFR 30–44 ml/min/1.73 m2, G4 – GFR 15–29 ml/min/1.73 m2, G5 – GFR < 15 ml/min/1.73 m2.
In the absence of evidence of kidney damage, neither GFR category G1 nor G2 fulfill the criteria for CKD [4, 5]. The GFR is considered the best overall index of kidney function. In the clinical practice GFR can be estimated based on different equation.
The modification of diet in renal disease (MDRD) and the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equations are the most widely used for estimating GFR in adult population [5].
The Cockroft-Gault formula should be used in patients taking medication which dosage depends on kidney function. Using MDRD or CKD-EPI equations for this purpose may lead to significant dosing errors in individuals whose body surface areas differs from 1.73 m2 [3]. For estimating GFR in pediatric population, “Bedside” Schwartz equation is the most common choice [5].
MDRD equation [5]:
eGFR ml/min/1.73 m2 = 175 × (Scr )–1.154 × (age in years)–0.203 × × [0.742 if female] × [1.212 if African American].
CKD-EPI equation [5]:
eGFR ml/min/1.73 m2 = 141 × min (Scr/κ, 1)α × × max(Scr/κ, 1)–1.209 × 0.993 (age in years) × × 1.018 [if female] × 1.159 [if black].
Cockroft-Gault equation [3]:
“Bedside” Schwartz equation [5]:
eGFR – estimated glomerular filtration rate, Scr – serum creatinine (mg/dl; κ – 0.7 for females and 0.9 for males, α – 0.329 for females and – 0.411 for males; min indicates the minimum of Scr/κ or 1; max indicates the maximum of Scr/κ or 1.
Thanks to contemporary therapeutic approaches, the incidence of renal insufficiency in RA has markedly diminished. Prior to the advent of methotrexate (MTX) treatment, targeted therapies, and biological interventions, CKD was diagnosed in as many as 37–5.07% of afflicted individuals [6].
According to published retrospective cohort studies in 2017, CKD occurs in approximately 24.5% of RA patients [7]. The “NinJa 2012” study [8] reported the following prevalence rates for the various stages of chronic renal disease: G1 – 25.4%, G2 – 55.9%, G3 – 17.5%, G4 – 0.8%, G5 – 0.2% [8].
The pathogenesis of CKD in RA patients is multifactorial. Renal impairment occurs as a consequence of drug-induced nephrotoxicity, comorbidities, and the direct effects of RA itself, including vasculitis and chronic inflammatory processes that contribute to secondary amyloidosis [9].
Nephrotoxic drugs known to significantly impact renal function encompass nonsteroidal anti-inflammatory drugs (NSAIDs) and certain previously employed and considered then as a disease-modifying antirheumatic drugs (DMARDs) such as penicillamine, gold salts, and cyclosporine, which presently hold limited significance or no longer feature in the therapeutic strategy for RA [10, 11].
The pathology induced by therapy and the disease can affect almost all kidney structures such as: glomeruli, vessels, tubules, and interstitium [6]. According to the MATRIX study [12], in the course of RA, proteinuria, hematuria, and leukocyturia are present in 16.2%, 17.2%, and 20.2% of patients, respectively [12].
There are several types of renal manifestations in RA such as: glomerular nephritis (mesangial, membranous, minimal change disease, focal segmental glomerulosclerosis), interstitial nephritis, necrosis of renal papillae, rheumatoid vasculitis, secondary amyloidosis of the kidneys, and CKD caused by cardiovascular system disorders (hypertension, diabetes, and advanced age). It is worth emphasizing that currently CKD in RA is more often associated with cardiovascular risk factors than uncontrolled disease activity [6].
Furthermore, special attention is required when glomerulonephritis in RA is accompanied by nephrotic syndrome. In cases of reduced total protein and albumin concentrations in the serum, not only does the concentration of free drug increase, thereby increasing the risk of adverse treatment effects, but the absorption of the drug is also impaired due to mucosal edema in the gastrointestinal tract.
An additional challenge in the presence of nephrotic syndrome is susceptibility to infections and an increased risk of thromboembolic events [3]. It is also worth emphasizing that general inflammation control significantly contributes to the inhibition of CKD progression in RA [13].
Treatment of rheumatoid arthritis
The aim of RA treatment is to achieve remission according to the criteria set by the American College of Rheumatology (ACR) and the European Alliance of Associations for Rheumatology (EULAR, formerly European League Against Rheumatism) [9–11].
Therapeutic effects are obtained through the immediate implementation of pharmacological treatment, rehabilitation involving both kinesiotherapy, physiotherapy, psychological support, and potential surgical interventions considered in cases where conservative treatment proves ineffective.
The foundation of pharmacological treatment consists of DMARDs, which are available in synthetic (sDMARDs) and biological forms (bDMARDs) (Table I). Additionally, NSAIDs are used for symptomatic relief, and in cases of contraindications to the NSAIDs, paracetamol (acetaminophen) and selected opioids can be considered. Another therapeutic option is also short-term (up to 3 months) oral administration or single parenteral dose of glucocorticosteroids (GCs), which should be applied when initiating or modifying treatment with conventional sDMARDs [6, 11].
Table I
In exceptional cases, in the event of significant disease exacerbation or the occurrence of certain organ complications, intravenous pulses of GCs may be administered for a few days [14].
Conventional synthetic disease-modifying antirheumatic drugs
Methotrexate is a folate antagonist that acts by inhibiting DNA synthesis and indirectly RNA and protein synthesis as well. In the doses using for the treatment of RA, the MTX block the enzyme 5-aminoimidazole-4-carboxamide ribonucleotide transformylase, leading to anti-inflammatory effects by increasing adenosine concentrations [15].
The elimination of the drug is mainly through the kidneys. Impaired kidney function can result in increased serum concentrations of the drug [16]. Methotrexate is the first-line treatment for RA [11]. Rheumatoid arthritis therapy requires low doses of MTX, which contributes to its high safety profile. However, it has been proofed that renal impairment is a significant concern in the population of cancer patients who receive high doses of MTX.
A study by Hayashi et al. [17], published in 2020, evaluating estimated glomerular filtration rate (eGFR) in three groups categorized based on the administered MTX dose, did not show a significant correlation between worsening renal function and a dose of < 12 mg weekly.
According to summary of product characteristics, renal function should be assessed before initiating MTX treatment and monitored during the therapy. The frequency of renal function assessment should be increased in the following cases: at the beginning of treatment, after dose changing, in situations of increased risk of elevated levels of MTX in the blood (i.e. dehydration, impaired renal function, administration of additional medications or increasing the dose of other concomitant medications.
However, the authors do not provide the required frequency of renal function assessment. In case of renal failure, concomitant use of MTX and non-steroidal anti-inflammatory drugs is inadvisable [18–20].
It is recommended to reduce the prescribed dose by 50% in patients with creatinine clearance (CrCl) < 60 ml/min, and the use of the drug is contraindicated in patients with CrCl < 30 ml/min [19].
Neither standard hemodialysis nor peritoneal dialysis increases the elimination of MTX. However, the elimination of MTX can be achieved by intensive, intermittent high-flow hemodialysis [18–20].
Data on the safety of MTX during dialysis vary depending on the source. According to Lizakowski [21] 50% of standard dose should be administrated 12 hours before the procedure of hemodialysis. Usage of MTX in peritoneal dialysis is contraindicated.
It is worth emphasizing that cases of MTX toxicity, including death, have been reported in patients being on either hemodialysis or peritoneal dialysis. Avoiding use of MTX in such cases seems reasonable [22, 23]. In a patient undergoing continuous renal replacement therapy (CRRT) dose of MTX should be reduced by 50% [21].
Leflunomide (LEF) is a substance with immunomodulatory, immunosuppressive, antiproliferative, and anti-inflammatory properties. Approximately 95% of the drug undergoes conversion to an active metabolite called teriflunomide, which is responsible for its therapeutic effects. The elimination of LEF is slow, occurring through urine and feces, with a half-life of approximately 2 weeks [24].
Leflunomide, along with sulfasalazine (SSZ), is a preferred choice of sDMARD when MTX is contraindicated [11]. According to the product characteristics of LEF containing medicinal products, dose adjustment is not necessary for patients with “mild renal impairment”. “Moderate and severe renal impairment” is contraindicated due to limited clinical experience in this patient group.
The descriptive names used to define the stages of renal impairment are not specified by the manufacturer. The active metabolite of LEF (A771726, teriflunomide) cannot be eliminated either by hemodialysis or peritoneal dialysis [24].
Sulfasalazine exhibits bacteriostatic, anti-inflammatory, and immunosuppressive effects. Under the influence of the intestinal bacterial flora, the drug is metabolized to sulfapyridine and mesalazine (5-aminosalicylic acid). Approximately 30% of mesalazine is excreted through the kidneys, while sulfapyridine, metabolized in the liver, is eliminated in both feces and urine [25].
There is insufficient data to determine the safety of SSZ in patients with CKD. Additionally, the drug manufacturer advises exercising caution in renal insufficiency. It is also recommended to monitor urine in this patient group, initially 1–2 times per month during the early treatment period, and then every 3–6 months. Summary of products characteristic do not provide any information about SSZ dosage during dialysis [26].
However, retrospective study on 8 RA patients suggests that SSZ may be efficacious and tolerated on hemodialysis at low doses (500 to 1000 mg daily) with careful monitoring. There is no safety data available for doses > 1 g/day [27]. Further research on this topic is necessary.
Chloroquine (CQ) and hydroxychloroquine (HCQ) are antimalarial drugs that, due to their anti-inflammatory properties, are also used in rheumatology. The exact mechanism of action in the treatment of systemic connective tissue diseases and conditions associated with photosensitivity is not fully understood.
Approximately 50% of CQ binds to plasma proteins. Elimination occurs through the kidneys in 50–60% of cases, either in unchanged form or as the active metabolite, desethylchloroquine. The half-life of CQ ranges from 10 to 60 days, while desethylchloroquine has a half-life of approximately 15 days [28].
There are no specific dosage adjustments in RA patients with renal insufficiency provided in the manufacturer’s labeling. The only specific recommendation refers to patients treated for malaria [29]. Hemodialysis, peritoneal dialysis, or exchange transfusion have not been shown to be of value in treating CQ poisoning [29].
Hydroxychloroquine is metabolized by the liver and approximately 25% is excreted in feces and up to 30% in urine. During short-term therapy, dose modification of HCQ is not required in patients with renal impairment, although dose reduction may be necessary for long-term treatment [6]. Renal insufficiency can intensify the toxic effects on the retina and heart, which may necessitate dose reduction. Furthermore, HCQ is not removed during dialysis [30].
Gold salts currently have marginal significance in the treatment of RA. It is important to emphasize that they exhibit high nephrotoxicity, and the occurrence of proteinuria is an absolute contraindication to continuing treatment. Membranous glomerulonephritis is the most common form of kidney damage associated with gold salts [9]. Systemic administration of gold salts should be avoided in patients with renal insufficiency [31].
The drugs discussed are summarized in Table II [18, 20–23, 25, 26, 28, 29, 31–35].
Table II
Dose modification of selected conventional synthetic disease modifying antirheumatic drug in renal insufficiency [18, 20–23, 25, 26, 28, 29, 31–35]
| csDMARD | Dose modification in renal insufficiency |
|---|---|
| Methotrexate | CrCl ≥ 60 ml/min – NDAR [18] CrCl 30–59 ml/min – reduction of dose by 50% [18] CrCl < 30 ml/min – contraindicated [18] |
| Hemodialysis: Avoid use. Cases of methotrexate toxicity (including death) have been reported [22, 23] Peritoneal dialysis: Avoid use. Cases of methotrexate toxicity (including death) have been reported [22, 23] CRRT: Reduction of dose by 50% [34] | |
| Leflunomide | “Mild” RI – NDAR [21] “Moderate and severe” RI – contraindicated [21] There are no specific dosage adjustments provided in the manufacturer’s labeling [21] |
| Hemodialysis: NDAR, use with caution [31, 32] Peritoneal dialysis: NDAR, use with caution [33] CRTT: ND, use with caution [33] | |
| Sulfasalazine | Caution should be exercised in patient with renal insufficiency [25] |
| Hemodialysis: Doses up to 1 g/day have been well tolerated, there is no efficacy or safety data available for doses > 1 g/day [26] Peritoneal dialysis: Doses up to 1 g/day have been well tolerated, there is no efficacy or safety data available for doses > 1 g/day [26] CRTT: ND | |
| Hydroxycholorquine | In short-term treatment – NDAR [29] In long-term treatment – DAR [29] Warning: Renal insufficiency can intensify the toxic effects on the retina and heart, which may necessitate dose reduction [29] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | |
| Chloroquine | Dosage adjustment provided in the manufacturer’s labeling refers only to treatment of malaria Caution should be exercised in RA patient with renal insufficiency [28] |
| Hemodialysis: Reduction of dose by 50% [20] Peritoneal dialysis: Reduction of dose by 50% [20] CRTT: NDAR [20] |
Targeted synthetic disease-modifying antirheumatic drugs
Tofacitinib (TOFA) is a second-generation selective inhibitor of Janus kinases (JAK). Janus kinases include the following four tyrosine kinases: JAK1, JAK2, JAK3, and TYK2. Their role is the intracellular phosphorylation of signal transducers and activators of transcription enzymes (STATs) that affect gene expression, hematopoiesis, and immune system function.
The JAK-STAT signaling pathway is involved in the pathogenesis of autoimmune diseases, including RA. By blocking the phosphorylation process, TOFA reduces inflammation and modifies the course of the disease. Tofacitinib is metabolized in 70% in the liver, 30% is eliminated through the kidneys. Its half-life equals 3 hours, and in the extended-release form – 6 hours [36].
The dosing of JAK inhibitors in renal insufficiency varies depending on the source and the criteria used for mild, moderate, and severe renal insufficiency [6, 36–39]. The following data is derived from the drug characteristics provided by the manufacturers. In the case of TOFA, no dose adjustment is necessary for patients with “mild” or “moderate” renal insufficiency. For CrCl < 30 ml/min, the dose should be reduced by 50%, which means reducing the dose to 5 mg per day.
The dose for patients with normal kidney function is 5 mg twice daily. The manufacturer specifies that in patients with “severe” renal impairment (CrCl < 30 ml/min), this dose should be maintained even after hemodialysis. Furthermore, in patients with end-stage renal disease, the impact of dialysis on the total clearance of TOFA is relatively small [36].
The JAK inhibitors used in the therapy of RA also include baricitinib (BARI) and upadacitinib (UPA). For BARI the recommended dose is 2 mg once daily for patients with a CrCl ranging from 30 to 60 ml/min. Baricitinib should not be administered in patients with CrCl < 30 ml/ min [38].
Upadacitinib does not require dose adjustment in “mild” or “moderate” renal insufficiency. Caution should be exercised in patients with “severe” renal impairment, with a CrCl of 15–30 ml/min. The manufacturer also notes that the use of UPA has not been studied in patients with end-stage renal disease and therefore should be avoided in such cases [37].
Filgotinib (FILG) requires a dose reduction from 200 mg once daily to 100 mg once daily for a CrCl of 15 to < 60 ml/min. The drug has not been studied in patients with a CrCl < 15 ml/min, so its use is not recommended in end-stage renal disease. The paragraph dedicated to FILG overdose states that there is no data available regarding its removal during dialysis [40].
The selected tsDMARD are presented in Table III [35–37, 39].
Table III
| tsDMARD | Dose modification in renal insufficiency |
|---|---|
| Tofacitinib | CrCl ≥ 30 ml/min – NDAR (5 mg twice per day) [35] CrCl < 30 ml/min – reduction of dose by 50% (5 mg once a day) [35] |
| Hemodialysis: In patients with severe renal impairment the reduced dose should be maintained even after hemodialysis [35] Peritoneal dialysis: ND CRTT: ND | |
| Baricitinib | CrCl ≥ 60 ml/min – NDAR (4 mg one a day) [37] CrCl 30–< 60 ml/min – reduction of dose by 50% (2 mg once a day) [37] CrCl < 30 ml/min – ND, use is not recommended [37] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | |
| Upadacitinib | CrCl ≥ 30 ml/min – NDAR [36] CrCl 15–< 30 ml/min – caution should be exercised (15 mg once a day) [36] CrCl < 15 ml/min – ND, use is not recommended [36] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | |
| Filgotinib | CrCl ≥ 60 ml/min – NDAR (200 mg once a day) [39] CrCl 15–< 60 ml/min – reduction of dose by 50% (100 mg once a day) [39] CrCl < 15 ml/min – ND, use is not recommended [39] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND |
Biological disease-modifying antirheumatic drugs
The 2022 update of EULAR guidelines classify biological disease-modifying drugs into original biological DMARDs (boDMARDs) and biosimilar DMARDs (bsDMARDs). Among boDMARDs, we distinguish anti-cytokine drugs such as adalimumab (ADA), certolizumab pegol (CZP), etanercept (ETC), golimumab (GOL), infliximab (IFX), tocilizumab (TCZ), sarilumab (SARI), and non-anti-cytokine drugs such as abatacept (ABT) and rituximab (RTX) [11].
According to a cohort study published in 2018 [41], involving a population of 20,757 individuals diagnosed with RA, the administration of biological drugs was associated with a reduced risk of progressive CKD, particularly in patients with eGFR < 45 ml/min. Irrespective of the type of biological treatment used, a slowing down of renal failure was observed.
The authors emphasize that based on the observation made, a causal relationship between biological treatment and a reduced rate of glomerular filtration decline cannot be determine. Biological DMARDs can contribute to a reduction in renal incidents both directly, by limiting renal inflammation and epithelial dysfunction, and indirectly, through modification of the metabolic profile and increased physical activity, while improving joint function [41].
However, treatment-induced renal damage does occur in clinical practice. In 2014, Piga et al. [42] published the results of an analysis of 29 rheumatic patients identified through a literature review (26 patients) and their own single-center cohort studies (3 patients from a population of 707 individuals) who were diagnosed with biologic-induced kidney disease.
The studied group included 22 patients with RA, 5 with spondyloarthropathy, and 2 with psoriatic arthritis. Based on the clinical manifestations and histological findings of the collected cases, the authors classified biologic-induced kidney damage into:
vasculitis-associated glomerulonephritis (12 cases),
glomerulonephritis in the lupus-like syndrome (4 cases),
isolated autoimmune kidney damage (13 cases).
Tumor necrosis factor inhibitors (TNFi) were the most common cause of renal injury, with ETC accounting for over half of the reported cases of nephrotoxicity due to boDMARDs. It should be noted that treatment-induced renal damage is rare but not incidental [42].
Manufacturers of drugs containing ADA, CZP, GOL, IFX, ABT and RTX state that no pharmacokinetic studies have been conducted to assess their impact on renal function and provide no recommendations for patients with renal impairment [43–49].
Limited clinical experience has not shown a need for dose adjustment of ETC in patients with renal insufficiency. One of manufacturers of ETC states that although radioactively labeled ETC was administered to volunteers and detected in urine, no increase in its concentration was observed in patients with acute kidney injury [45].
The use of anakinra does not require dose adjustment in patients with CrCl 60–< 90 ml/min, but caution is advised in patients with CrCl 30–< 60 ml/min. In cases of CrCl < 30 ml/min, including in dialysis patients, the administration of recommended therapeutic dose should be considered every other day [50].
There is no need to adjust the dose of TCZ in patients with CrCl ≥ 50 ml/min. However, due to the lack of pharmacokinetic studies of TCZ in individuals with CrCl < 50 ml/min, the manufacturer recommends close renal monitoring in this patient group [51].
The summary of product characteristics of the medicinal product containing SARI indicates the absence of conducted “formal” studies regarding the pharmacokinetics of this drug in patients with renal impairment. Furthermore, the manufacturer states that there is no need to adjust the dose in patients with “mild” or “moderate” renal impairment, without defining these terms [52].
Dose modification of selected biological disease-modifying antirheumatic drugs in renal insufficiency are presented in Table IV [42–51].
Table IV
Dose modification of selected biological disease-modifying antirheumatic drugs in renal insufficiency [42–51]
| bDMARD | Mechanism of action | Dose modification in renal insufficiency |
|---|---|---|
| Adalimumab | TNF-α inhibitor | The use of the product in patients with renal insufficiency has not been studied. No recommendations can be made [42] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | ||
| Certolizumab | TNF-α inhibitor | The use of the product in patients with renal impairment has not been studied. No recommendations can be made [47] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | ||
| Etanercept | TNF-α inhibitor | Patients with renal insufficiency: NDAR – clinical experienced is limited [44] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | ||
| Golimumab | TNF-α inhibitor | The use of the product in patients with renal impairment has not been studied. No recommendations can be made [45] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | ||
| Infliximab | TNF-α inhibitor | The use of the product in patients with renal impairment has not been studied. No recommendations can be made [46] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | ||
| Rituximab | Monoclonal anti-CD20 antibody | The use of the product in patients with renal impairment has not been studied. No recommendations can be made [48] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | ||
| Tocilizumab | Anti-IL-6 receptor antibody | CrCl ≥ 50 ml/min – NDAR [50] CrCl < 50 ml/min – ND, strict monitoring of renal function is advised [50] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | ||
| Sarilumab | Anti-IL-6 receptor antibody | “Mild/moderate” RI – NDAR [51] “Severe” RI – ND [51] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | ||
| Anakinra | Anti-IL-1 receptor antagonist | CrCl 60–< 90 ml/min – NDAR [49] CrCl 30–< 60 ml/min – caution should be exercised [49] CrCl < 30 ml/min/dialysis – the administration of recommended therapeutic dose should be considered every other day [49] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | ||
| Abatacept | CD80/86-CD28 T cell co-stimulation modulator | The use of the product in patients with renal impairment has not been studied. No recommendations can be made [43] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND |
Nonsteroidal anti-inflammatory drugs and other selected analgesic medications
Nearly half of patients with RA use NSAIDs for the purpose of temporary, and less frequently regular, pain and stiffness control. Despite numerous adverse effects on the cardiovascular, gastrointestinal, and renal systems, NSAIDs continue to be a valuable class of drugs for alleviating RA symptoms [1].
The anti-inflammatory action of NSAIDs arises from the inhibition of prostaglandin synthesis by inhibiting cyclooxygenase (COX) enzymes. This leads to the blockade of afferent arteriolar vasodilation, reversible ischemia, decreased glomerular filtration, and ultimately potential kidney damage.
Reduced prostaglandin production may also result in tubulointerstitial nephritis, type 4 renal tubular acidosis with hyperkalemia, and necrosis of renal papillae. NSAIDs may also cause interstitial nephritis due to allergic reactions [6].
Continuation of NSAID treatment in the presence of worsening renal function may lead to further progression of CKD [6]. Dosage modification recommendations for patients with renal insufficiency are individualized for each NSAID.
The ACR guidelines advise caution when using NSAIDs in patients with a CrCl ranging from 30 ml/min to 60 ml/min, and they should be avoided when CrCl is < 30 ml/min [53].
According to a study by Jankovic et al. [54], the use of NSAIDs is contraindicated in patients with end-stage renal disease [54]. The result of a prospective cohort study by Möller et al. [55], indicate that NSAIDs do not have a negative impact on renal function in patients with a glomerular filtration rate (GFR) ≥ 30 ml/min [55].
An alternative to NSAIDs for patients intolerant or contraindicated to their use include paracetamol (acetaminophen) and opioid medications such as tramadol [3]. Dosage adjustments for renal insufficiency are presented in the table IV–VI [23].
Table V
| Symptomatic medication | Dose modification in renal insufficiency |
|---|---|
| NSAIDs (general recommendations*) | CrCl ≥ 60 ml/min – NDAR [52] CrCl 30–60 ml/min – caution should be exercised [52] CrCl < 30 ml/min – use should be avoided [52] |
| Hemodialysis, peritoneal dialysis, CRTT* | |
| Paracetamol (acetaminophen) | CrCl > 30 ml/min NDAR – 0.5 mg every 6 hours [55] CrCl 10–30 ml/min – 0.5 g every 6 hours [55] CrCl < 10 ml/min – 0.5 g every 8 hours [55] |
| Hemodialysis: 0.5 g every 8 hours [20] Peritoneal dialysis: 0.5 g every 8 hours [20] CRTT: 0.5 g every 6 hours [20] | |
| Tramadol | CrCl ≥ 30 ml/min – IR/ER – NDAR [56] CrCl < 30 ml/min – IR – increase dosing interval to every 12 hours (maximum daily dose of 200 mg); ER – should be avoided [56] |
| Hemodialysis: IR – lower initial doses and an extended dosing interval. Do not exceed 50 mg twice daily, a uremic state may lower seizure threshold. ER – should be avoided [57–59] Peritoneal dialysis: IR – lower initial doses and an extended dosing interval. Do not exceed 50 mg twice daily, a uremic state may lower seizure threshold. ER – should be avoided [57–59] CRTT: Lower initial doses and an extended dosing interval. Do not exceed 50 mg twice daily, a uremic state may lower seizure threshold. ER – should be avoided [20, 57–59] |
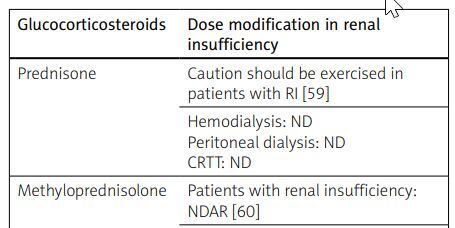
Table VI
| Glucocorticosteroids | Dose modification in renal insufficiency |
|---|---|
| Prednisone | Caution should be exercised in patients with RI [59] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | |
| Methyloprednisolone | Patients with renal insufficiency: NDAR [60] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND | |
| Dexamethasone | Patients with renal insufficiency: NDAR [61, 62] |
| Hemodialysis: ND Peritoneal dialysis: ND CRTT: ND |
The drugs discussed are summarized in Table V [20, 52, 55–59].
Glucocorticosteroids
In the EULAR 2022 guidelines, special attention has been given to clarifying the definition of “short-term GC therapy” in RA, which is defined as lasting up to 3 months. Glucocorticosteroids can be particularly helpful in so-called bridging therapies, at the initiation or modification of current csDMARD therapy [11].
Through various cellular and molecular mechanisms, GCs exhibit potent anti-inflammatory and immunosuppressive effects. In the treatment of RA, prednisone (PRD), methylprednisolone (MP), and dexamethasone (DXM) are primarily used. An inactive prednisone is converted into its active metabolite, prednisolone [14].
Prednisone is mainly metabolized in the liver, to a lesser extent in the kidneys. Excretion occurs primarily via bile, 1–5% excreted in the urine as inactive metabolites and 10–20% as PRD. The product characteristics of a medication containing PRD emphasize the need for caution when using it in patients with renal insufficiency [60].
Methylprednisolone exhibits stronger anti-inflammatory effects compared to PRD. It is used in the treatment of RA through intravenous, intra-articular, and oral administration. The manufacturer highlights the need for caution in patients with renal insufficiency and states that MP is dialyzable [61].
The selected glucocorticosteroids are presented in Table VI [59–62].
Conclusions
Chronic kidney disease in patients with RA is a consequence of the drugs nephrotoxicity, coexisting conditions, and the course of disease itself. Contemporary treatment strategies, primarily based on DMARDs with a high safety profile, have reduced the incidence of renal failure in the population of RA patients. However, it remains a problem for approximately 25% of patients.
Therefore, special attention should be paid to the potential need for dosage modifications of administered medications. Many drugs used in the therapy of rheumatic diseases have not been thoroughly studied for their safety in patients with reduced glomerular filtration, resulting in limited data in this area.
Moreover, product characteristics often use descriptive names for the stage of renal failure. Yet, although interpretation of these is essential to choose the required dose, they are not specified. The establishment of precise, transparent, and consistent dosage recommendations for antirheumatic drugs in CKD would significantly facilitate the care of patients with RA. However, further research is needed to achieve this goal.