Introduction
In May 2023, the World Health Organization (WHO) declared the end of the COVID-19 pandemic. However, this does not mean that people, including children, will not get sick with a coronavirus infection. COVID-19 has transitioned to the rank of seasonal diseases, with similar frequency and course characteristics as other viral illnesses.
Nevertheless, COVID-19 leaves a significant trail of symptoms and conditions under the umbrella of long COVID or post-COVID. In general, long COVID symptoms are found in at least 10–20% of individuals who have had a SARS-CoV-2 infection [1]. This means that more than 80 million people may experience long COVID symptoms, which not only have a substantial impact on healthcare but also may carry significant social and economic consequences [2].
Currently, there are several definitions of long COVID, and several terms are used for the same condition (Table I): “post-COVID-19 condition”, “post-COVID syndrome” (PCS), “post-acute sequelae of SARS-CoV-2 infection” (PASC), “long COVID”, “ongoing symptomatic COVID-19”.
Table I
Terms and definitions of post-acute sequelae of COVID-19
| Organization | WHO [4] | CDC [5] | NICE [6] |
|---|---|---|---|
| Term | Post-COVID-19 condition, long COVID | Post-COVID-19 condition, long COVID | Long COVID that includes ongoing symptomatic COVID-19 and post-COVID-19 syndrome |
| Last publishing date | December 7, 2022 | December 16, 2022 | November 3, 2022 |
| Definition | The continuation or development of new symptoms 3 months after the initial SARS-CoV-2 infection, with these symptoms lasting for at least 2 months with no other explanation | Umbrella term for the wide range of physical and mental health consequences experienced by some patients that are present four or more weeks after SARS-CoV-2 infection, including patients who had initial mild or asymptomatic acute infection | Ongoing symptomatic COVID-19 – signs and symptoms of COVID-19 from 4 to 12 weeks post-COVID-19 syndrome – signs and symptoms that develop during or after an infection consistent with COVID-19 12 weeks or more |
It is considered that long COVID is a widely used term, while PCS means health consequences that are due to all effects of COVID-19, including secondary and tertiary effects. The PASC covers the direct and indirect consequences of SARS-CoV-2 on human health [3–5].
Thus, despite updates in definitions around the same time (November–December 2022), there are certain discrepancies regarding the duration of symptoms presence – from 4 weeks according to the Centers for Disease Control and Prevention (CDC) definition [5] to 12 weeks or more according to the WHO [4] and National Institute for Health and Care Excellence (NICE) definitions [6]. However, these definitions mainly focused on adults.
For children and young people, the definition of post-COVID-19 condition (long COVID) was established using a Delphi process [7]. Post-COVID-19 condition in children and adolescents refers to the persistence of one or more physical symptoms for a minimum of 12 weeks after initial testing, which cannot be explained by an alternative diagnosis in a person with a confirmed history of SARS-CoV-2 infection. These symptoms may continue or develop after COVID-19 infection, fluctuate, relapse over time, and have an impact on everyday functioning [7].
SARS-CoV-2 infection can be determined through an antigen test, a PCR test, or an antibody test.
Long COVID encompasses not only a wide range of definitions and terms but also a diversity of symptoms. More than 200 symptoms associated with long COVID have been described [7, 8]. While most publications describing symptoms have focused on adults, common symptoms such as fatigue and headache have also been reported in children [8]. Additionally, lack of concentration and muscle pain were frequent symptoms observed in children [9].
Of course, the symptoms in children will vary depending on their age. Among pre-school children, the most commonly reported symptoms were fatigue, loss of smell, loss of taste, and muscle weakness, whereas for school children the most significant symptoms were loss of smell and taste, fatigue, respiratory problems, dizziness, muscle weakness, and chest pain [10].
Within the variety of post-COVID symptoms, four main phenotypes or clusters of patients have been identified: a cardiorespiratory phenotype, clusters with pain and musculoskeletal symptoms, a predominant anosmia phenotype, and a neuropsychiatric cluster [11].
Several hypotheses about the pathogenetic mechanisms of long COVID have been proposed and summarized [2, 9, 11]. Immune system dysfunction is one of the leading hypotheses in the development of long COVID. Key hypotheses include immune dysregulation, autoimmunity, insufficient immune response, chronic low-grade systemic inflammation, and superantigen production [2, 11]. Among other hypotheses, genetic and epigenetic factors, viral persistence, reactivation of latent viruses, endothelial dysfunction and coagulopathy, dysfunctional neurological signaling, and microbiome dysbiosis were defined [2, 11].
However, it should be noted that these mechanisms often intertwine with each other. Attempts have been made to determine the involvement of specific mechanisms in certain long COVID phenotypes [11].
Autoimmunity is defined as the presence of antibodies or T lymphocytes that react with self-antigens [12]. However, the presence of autoantibodies may be found in all individuals and increases with age, but it does not always have pathogenic consequences. Polyreactive autoantibodies that recognize multiple host antigens are present throughout life and play an important positive role as they clear apoptotic remnants. Autoimmune diseases only occur when there is a disruption in immune regulation mechanisms, leading to self-reactivity and tissue damage [12].
The question of the role of SARS-CoV-2 infection in the development of autoimmune diseases is still a topic of debate, with arguments for and against [13, 14]. The role of the presence of autoantibodies in the development and course of long COVID and their correlation with specific long COVID phenotypes has not been fully determined. Moreover, the leading factors transforming autoimmunity into autoimmune disease are not yet established. Genetic predisposition may also play a role in this case, as indicated in certain studies [15]. Additionally, there are many questions regarding the duration of the presence of antibodies and the monitoring strategy for individuals, whether children or adults, when antibodies are present.
Material and methods
A case study presentation as a basis for the discussion was used. Here we present two cases of long COVID in a 13-year-old boy and a 14-year-old girl. The boy exhibited pronounced weakness, arthralgia, myalgia, rashes, and Raynaud’s syndrome, which mimics autoimmune diseases, particularly idiopathic inflammatory myopathies. Borderline values of antibodies to Mi-2, SRP 54, and PL-12 were observed. In contrast, in the girl, clinical manifestations were limited to periodic headaches and low-grade fever, but the level of antinuclear antibodies was high, and there were deviations in the lymphocyte subpopulations.
We performed a systematic search in the PubMed Medline database using the specified search terms: “long COVID”, “post COVID”, “autoimmunity”, “autoimmune diseases” and “children”. We included relevant full-text articles in English that were published between January 2020 and July 2023.
The selected studies focused on immune dysregulation, autoimmunity, their association with clinical phenotype, and treatment options in patients with long COVID. These articles were chosen for in-depth analysis to gain insights into the relationship between long COVID and autoimmunity in children.
The aim of this article was to analyze the potential role of autoimmunity in the development of long COVID symptoms and explore treatment approaches, particularly in children. The review focused on understanding how immune dysregulation and autoimmunity may contribute to long COVID clinical manifestations in pediatric patients and investigated potential therapeutic strategies.
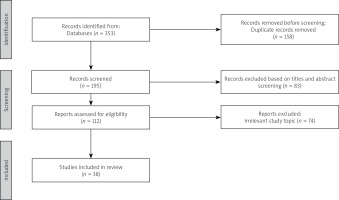
Overall, a total of 353 publications were initially identified using the specified search terms. After excluding duplicates and records irrelevant to the study topic, 38 relevant publications were included in the final analysis. The study selection process is shown in Figure 1.
The review of the literature indicated that infections, including SARS-CoV-2, are often a common cause of autoimmunity, but in most cases, it is self-limiting. The presence of autoantibodies does not always indicate the presence of a specific autoimmune disease but requires careful observation. The autoimmune hypothesis remains one of the main ones regarding the development of long COVID. Moreover, it has been shown that both anti-SARS-CoV-2 antibodies and specific autoantibodies may be associated with diverse patterns of post-acute sequelae of COVID-19.
Despite the abundance of clinical research and some successes in identifying therapeutic approaches for the treatment and rehabilitation of patients with long COVID, the treatment and rehabilitation tactics for pediatric patients remain uncertain, especially for children with autoimmune manifestations.
Case description
Descriptions of the cases are summarized in Table II and Table III.
Table II
Clinical and laboratory characteristics of patients
Table III
Immunological parameters and autoantibodies of the patients
Case 1
A 13-year-old boy sought medical help due to pronounced weakness, shortness of breath during physical exertion, pain in the lower limbs, both in the joints and muscles, periodic appearance of spotty rashes on the body, discoloration of the hands and feet, which worsened in cold conditions, and dizziness when changing body position (Table II). These symptoms had been observed for approximately 3 months. He was examined and treated at the outpatient department, but significant improvement was not noted.
The complaints arose after a viral infection (probably COVID-19) that the boy had in February 2023. Prior to the illness, the boy was active, heavily engaged in sports, and participated in competitions. Mild Raynaud’s syndrome symptoms were observed from the beginning of winter but significantly worsened after the episode of infection. In the medical history, there was myositis at the age of 6. In October 2020, the patient had COVID-19 pneumonia, confirmed by PCR for the first time. No significant health deviations were observed after recovering from the previous episode of SARS-CoV-2 infection.
During the examination, the boy’s weight was 42 kg (Z-score – 0.54), height was 135 cm (Z-score – 2.85), and BMI was 23.1 (Z-score – 1.26). Pale skin color and café-au-lait spots on the lower limbs were observed during the objective examination. The skin on the hands and feet appeared red, moist, and cold. The heart’s activity was rhythmic, with audible heart sounds, and a systolic murmur with an intensity of 2/6 was detected at the apex. The heart rate was 76 beats per minute in the horizontal position and 92 per minute in the vertical position. The blood pressure was 120–130/80 mmHg. Vesicular breath sounds were noted in the lungs. No gastrointestinal abnormalities were found. The joints appeared normal, with a full range of motion, and muscle strength in the upper and lower extremities was rated 5/5.
In the laboratory examination, no significant changes were found in the complete blood count (CBC) and coagulogram. The levels of C-reactive protein (CRP), lactate dehydrogenase, creatine phosphokinase, aspartate aminotransferase, as well as other biochemical markers were within the normal range. The vitamin D level was 37.7 ng/ml, which was within the normal range. Slight deviations in the CD3+, CD4+, and CD19+ lymphocyte subpopulations were observed, while the levels of immunoglobulins were normal.
The examination for SARS-CoV-2 antibodies using the ELISA (enzyme-linked immunosorbent assay) method showed positive IgM + IgA antibodies at 13.5 U and IgG antibodies at 14.3 U (positive results when the value is above 1.0 U). A month later, IgM became negative, and IgG decreased to 8.04 U. In the examination for antibodies to Epstein-Barr virus (EBV) and cytomegalovirus (CMV), only IgG antibodies to CMV were positive.
The investigation of the thyroid gland revealed an elevated level of thyroid-stimulating hormone (TSH) at 5.09 mIU/ml (normal range 0.3–4.5 mIU/ml) with normal levels of T3, T4, and thyroid peroxidase-antibodies, indicating minimal thyroid insufficiency.
The electrocardiogram (ECG) and 24-hour ECG monitoring showed sinus arrhythmia and early repolarization in the ventricles. The echocardiogram detected minimal mitral regurgitation. Blood pressure monitoring did not show significant deviations.
Considering the pronounced weakness and dizziness, central nervous system pathology was ruled out based on magnetic resonance imaging, which did not reveal significant changes. Given the presence of Raynaud’s syndrome, arthralgia, myalgia, and the periodic appearance of spotted rashes, we also thought about systemic autoimmune diseases. Antinuclear antibodies (ANA) were negative, as were the indicators in the standard autoimmune panel. The myositis-specific antibody panel showed borderline values for Mi-2, SRO 54, and PL-12 (Table III).
Electromyography did not reveal any changes characteristic of myositis. Capillaroscopy showed a reduction in the number of capillaries and their tortuosity, consistent with microangiopathy and secondary Raynaud’s syndrome.
Symptomatic treatment of Raynaud’s syndrome and involving a psychologist in the treatment positively affected the course of the disease.
Case 2
A 14-year-old girl complained of low-grade fever and headache. These symptoms had periodically bothered the girl since September 2020 when she first had COVID-19. The course of SARS-CoV-2 infection was moderate, with symptoms of fever, runny nose, and cough. However, low-grade fever and headache persisted for more than 3 months after the initial episode of infection. The second episode of COVID-19 occurred in January 2021 with mild symptoms. Subsequent episodes of low-grade fever and headache occurred every 6 months and lasted for several months. On physical examination, no significant changes in internal organs were found. Complete blood count showed lymphopenia (1.35 × 109/l) and eosinophilia (18%). Determination of IgM to SARS-CoV-2 was carried out at each episode of fever and headache and it remained positive for 2 years.
The immunological investigation revealed moderate decreases in absolute values of CD3+, CD4+, and CD19+ cells, and significant decreases in relative and absolute values of CD8+ cells. The immunoregulatory index was elevated. Levels of IgA, IgM, and IgG were within normal limits, but IgE was significantly elevated (1,500–3,700 IU/ml). ANA titer was 1 : 1,000, and IgG RNP 70 was also positive (Table III). Helminthic infestation was ruled out. The girl did not exhibit any pronounced allergic manifestations. Genetic testing of the panel of primary immunodeficiencies did not reveal any clinically significant variants.
The treatment included periodic intake of medications to alleviate headaches.
Discussion
The first case presented demonstrated the pheno-type of long COVID with pain and musculoskeletal symptoms, which is often associated with autoimmune disorders and may mimic systemic connective tissue diseases. The second case showed recurrent episodes of fever and headache every 4–6 months after the initial COVID-19 episode. It was associated with persistent positive IgM to SARS-CoV-2, a positive titer of ANA (1 : 1,000), and positive IgG RNP 70, without any other clinical signs of autoinflammatory disease. Therefore, the determination of autoantibodies may not always provide a definitive answer regarding the presence of an autoimmune process or disease. Also, it is not clear whether these antibodies affect the course of the disease or if they are just a manifestation of pronounced and prolonged inflammation [16].
The role of viruses as triggers for the development of autoimmune diseases has been studied for a long time. In PubMed there are over 19,000 publications dating back to 1962 that show results related to “viruses and autoimmunity”. Before the COVID-19 pandemic, the most significant attention was given to herpesviruses, Coxsackie B virus, rotavirus, and influenza A viruses as potential triggers for autoimmunity [17, 18]. However, the exact mechanisms by which viruses trigger autoimmunity are still not fully understood.
The molecular mimicry hypothesis is the most popular explanation, suggesting that viral antigens may share similarities with host antigens, leading to the production of antibodies that can mistakenly damage host tissues [15–17]. Other mechanisms, such as epitope spreading [19], bystander activation [20], and immortalization of infected B cells [21] are also discussed in the context of viral-triggered autoimmunity [18]. Viruses can also produce superantigens, which are not restricted by the major histocompatibility complex (MHC) and can significantly activate CD4 and CD8 T-cells [17]. Studies have shown that viral infections can either induce or protect against autoimmune pathologies depending on various factors, including genetics, the immune response, the type of virus strain, viral load, and the time of infection [18].
These factors play a crucial role in determining the outcome of viral infections and their potential contribution to autoimmunity.
Epstein-Barr virus plays an important role in the pathogenesis of systemic autoimmune diseases [22, 23]. However, anti-CMV, anti-EBV, and anti-herpes simplex virus type 1 (HSV-1) IgG levels were inversely correlated with transglutaminase type 2 antibody levels, suggesting a potential protective impact of these viruses in the pathogenesis of celiac disease [24]. Certain infections can shift the immune response from Th1 to Th2, leading to the suppression of Th1-derived immune diseases [25]. Similarly, helminth parasites have shown protective effects against autoimmunity through innate type 2 cytokines, as demonstrated in patients with multiple sclerosis [26].
The role of SARS-CoV-2 in the development of autoimmune diseases is still under discussion [27]. Among different SARS-CoV-2 proteins, the spike protein has been identified as a potential epitopic target for biomimicry-induced autoimmunity [28]. Most reports focused on the development of organ-specific conditions, such as Guillain-Barré syndrome, after SARS-CoV-2 infection. Over the past 4 years, more than 1,000 publications related to “Guillain-Barré syndrome and COVID-19” have been presented in PubMed, indicating the potential role of SARS-CoV-2 in the development of Guillain-Barré syndrome [27, 29].
Other studies have reported associations between SARS-CoV-2 infection and vasculitis, arthritis, idiopathic inflammatory myopathies, systemic lupus erythematosus, sarcoidosis, systemic sclerosis, and adult-onset Still’s disease [30–32].
Immune dysregulation can lead to the development of autoimmune phenomena, resulting in the production of autoantibodies and potentially leading to the onset of rheumatic autoimmune diseases [32]. Sacchi et al. [33] support the hypothesis that SARS-CoV-2 infection is associated with autoimmunity markers. In patients with COVID-19, ANA, antineutrophil cytoplasmic antibodies (ANCA), and anti-Saccharomyces cerevisiae antibodies (ASCA) were detected, and their presence negatively affected the outcome of COVID-19. Levels of these antibodies were significantly higher in COVID-19 patients compared to healthy subjects. The study also revealed that patients experiencing new autoimmune phenomena had the poorest viral disease prognosis and outcome [33].
Other studies also showed the activation of autoimmune mechanisms during COVID-19 [34, 35]. Antibodies against type I interferon (IFN) are known to play a significant role in the development of severe infections [36]. However, most of these studies were conducted during the acute phase of COVID-19, and mainly in adult patients. The duration of autoantibodies in the blood and their role in the development of long COVID remain unclear. In a particular study, it was observed that 43.6% of patients had ANA titers ≥ 1 : 160 at 12 months after the onset of COVID-19 symptoms [37]. Additionally, the frequency of neurocognitive symptoms was significantly higher in this group of patients compared to those with titers < 1 : 160. The authors concluded that autoimmunity may be a contributing factor in the etiology of long COVID.
In our patient (case 2), the ANA titer had remained above 1 : 1,000 for over 2 years. The symptoms of long COVID are associated with recurrent fever and headache. Further research is needed to better understand the persistence and potential implications of autoantibodies in the context of long COVID.
Guest et al. [38] suggested that persistent neurological and neuropsychiatric symptoms in long COVID may be a result of autoimmune-mediated systemic and brain-vascular dysregulation, leading to circulatory disorders, fatigue, cognitive impairment, depression, and anxiety. Specific antibody diagnostics and careful clinical and psychological examinations are required for accurate diagnosis.
Immune dysregulation is a major hypothesis not only in severe COVID-19 but also in long COVID, influencing the symptoms and duration of the condition [11]. Files et al. [39] observed prolonged immune dysregulation after SARS-CoV-2 infection, with T cell activation/exhaustion markers (PD-L1 and TIGIT) remaining elevated in both hospitalized and nonhospitalized individuals. B cells also demonstrated a similar pattern of activation/ exhaustion. Interestingly, T cell exhaustion was observed even one year after infection, though the mechanisms of T lymphocyte dysfunction and their association with the development of long COVID remain unclear [11].
Changes in the immune system during SARS-CoV-2 infection are associated with both acquired and innate immunity [40]. Patients with long COVID showed activated innate immune cells and elevated expression of type I interferon (IFN-β) and type III IFN (IFN-λ1), which remained high at 8 months after infection [41].
Cytokine storm in patients with COVID-19 leads to endothelial inflammation, microvascular thrombosis, and multiple organ failures [42]. Similarly, patients with long COVID also demonstrated elevated levels of interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), and IFN-γ inducible protein-10 (IP-10) [43]. Interstitial lung changes at 3 to 6 months after recovery were associated with increased chemokines, proteases, and markers of neutrophil extracellular traps, as well as increased type 1 interferon signaling [44].
The relationship between the development of long COVID and the quantity of antibodies against SARS-CoV-2 infection remains unclear. Some studies suggest that neutralizing antibodies after COVID-19 or vaccination may protect from severe SARS-CoV-2 infection [45]. Therefore, it is plausible to assume a lower possibility of long COVID, but this condition can still develop even after mild cases of COVID-19 [11, 46]. On the other hand, disturbances in the humoral response may lead to uncontrolled immune activation and the development of severe COVID-19 [11].
Su et al. [46] found that anti-SARS-CoV-2 antibodies and specific autoantibodies were associated with different patterns of post-acute sequelae of COVID-19. Patients with neurological symptoms showed slightly higher levels of anti-SARS-CoV-2 nucleocapsid protein IgG, while post-COVID symptoms related to the digestive system and sputum production were associated with elevated levels of multiple autoantibodies 2–3 months after the onset of initial symptoms.
Autoantibodies to IFN-α2 were related to respiratory-viral post-COVID condition. The research suggested that autoantibody levels in acute disease may serve as biomarkers for certain long COVID phenotypes [47]. Moreover, in acute disease, inflammation biomarkers, including IFN-γ, CRP, and IL-6, were positively correlated with autoantibodies in the post-acute sequelae of COVID-19, suggesting a connection between autoantibodies, hyperinflammation, and long COVID. However, another study reported a low prevalence of anti-IFN antibodies in the post-acute period [48].
Bowe et al. [49] reported an increasing risk of long COVID after the second and third infection, even in double-vaccinated and triple-vaccinated individuals. In our case 1, long COVID symptoms developed after the second episode of SARS-CoV-2 infection, which was significantly milder than the first episode and did not require hospitalization. However, in case 2, long COVID symptoms developed after the first episode of COVID-19.
Overall, infections are often a trigger for autoimmunity, but in most cases, the immune response is self-limiting. Sometimes, even in the presence of organ pathology, it can be challenging to determine whether the damage is due to autoimmunity. The presence of autoantibodies, including ANA, must always be evaluated in association with clinical features because approximately 25% of the healthy population may have ANA antibodies [50, 51].
The therapeutic approach for patients with long COVID, especially for children, depends on the severity of their symptoms and individual clinical characteristics. Some patients may only require observation and monitoring, while others may need active management [52].
Most therapeutic strategies for long COVID are aimed at alleviating symptoms [53]. However, it is crucial to conduct a thorough and comprehensive evaluation of symptoms and clinical signs to promptly recognize conditions that may impact the patient’s prognosis [2].
Diagnosis should be made at the primary care level, and when warning signs are detected, patients should be referred to appropriate specialists or interdisciplinary care facilities [54]. Numerous clinical trials have been registered to investigate the impact of different pharmacological agents such as anti-inflammatory drugs, steroids, anticoagulants, and antidepressants, as well as dietary and herbal supplements, vitamins, cell-based treatments, and lifestyle interventions on reducing long COVID symptoms [2].
Treatment modalities depend on the specific phenotype of long COVID. Lifestyle management, including pacing, energy optimization, and regular rests, along with low-dose naltrexone, have shown good efficacy among patients with neurological phenotypes, such as myalgic encephalomyelitis/chronic fatigue syndrome [1, 55].
AXA1125, a novel metabolic modulator composed of 5 amino acids and N-acetylcysteine, has demonstrated the ability to reverse mitochondrial dysfunction, improve energetic efficiency through increased fatty acid oxidation, restore cellular homeostasis, and reduce inflammation. In patients with fatigue-predominant long COVID, oral administration of AXA1125 twice daily for 4 weeks resulted in greater symptomatic improvement compared to a placebo [55]. Coenzyme Q10, D-ribose, and low-dose aripiprazole may also be useful in managing fatigue in some patients [1].
Increasing salt and fluid intake, intravenously administered salt, compression stockings, β-blockers, low-dose fludrocortisone, pyridostigmine, and desmopressin are useful in managing dysautonomia with postural hypotension in patients with long COVID [1, 56].
The combination of nirmatrelvir with ritonavir has shown potential effectiveness in relief of long COVID symptoms [57], although other studies did not find evidence to support efficacy of nirmatrelvir in combination with ritonavir (a combination of a SARS-CoV-2 main protease inhibitor [MPro] and an inhibitor of CYP3A4 metabolism) in long-term consequences of COVID [58].
A randomized, placebo-controlled clinical trial demonstrated the effectiveness of metformin in preventing long COVID, whereas ivermectin or fluvoxamine did not show efficacy [59].
Antivirals such as valaciclovir, famciclovir, valganciclovir, and others may be used in cases of viral persistence and reactivations of EBV, CMV, and varicella-zoster virus [1].
Intravenous immunoglobulin may be an option for patients with long COVID and immune dysregulation [1].
Data regarding the therapeutic approach for auto-immune manifestations in long COVID are limited. A promising direction is the use of the molecule BC007 in patients with long COVID and its potential influence on autoimmunity [1]. Wallukat et al. [60] reported that more than 90% of patients with long COVID had autoantibodies against G protein-coupled receptors. BC007 has been used to neutralize autoantibodies against G protein-coupled receptors, and 4 patients treated with BC007 at the University of Erlangen achieved remission of long COVID symptoms, particularly cardiac and neuro-logical symptoms, in a short time. Clinical trials of this drug are currently being conducted in clinics in Munich and Berlin.
Conclusions
Our study has shown that there is substantial evidence linking SARS-CoV-2 infection to the production of autoantibodies and the development of autoimmunity. Immune dysregulation and autoimmunity are considered to be among the leading mechanisms in the development of long COVID. Our clinical cases demonstrate that the clinical manifestations of long COVID may mimic autoimmune diseases, and the determination of autoantibodies may not always provide a definitive answer regarding the presence of an autoimmune process.
Only a comprehensive evaluation of clinical symptoms and thorough objective examination can confirm or exclude systemic connective tissue diseases after a previous SARS-CoV-2 infection.
The management approach for children with symptomatic long COVID and the presence of autoantibodies remains uncertain and requires further research.