Introduction
Sjögren’s syndrome (SS) is an autoimmune disorder characterized by the inflammatory involvement of lacrimal and salivary glands, leading to xerostomia and/or xerophthalmia and eventually to the progressive atrophy of the glandular parenchyma. Since no specific drug is approved for SS, the treatment is usually only symptomatic.
Sjögren’s syndrome is typically divided into a primary form, usually affecting women (female : male ratio 14 : 1) and a secondary SS, typically associated with other autoimmune disorders, such as rheumatoid arthritis (RA) and systemic lupus erythematous (SLE) [1, 2].
Salivary glands biopsy is a useful and widely employed tool for the diagnosis of SS, but also plays a role in the diagnosis of many other diseases, such as amyloidosis, sarcoidosis and lymphomas. Several approaches are described, but minor salivary glands biopsy (MSGB) is the most commonly performed in clinical practice.
Autoimmunity evaluation is of paramount importance in the diagnosis of SS, since both anti-SSA/Ro presence and a positive MSGB, play a major role in American College of Rheumatology/European Alliance of Associations for Rheumatology (ACR/EULAR) classification criteria for primary SS [3].
The main objective of our study was to evaluate the diagnostic role of MSGB among patients presenting ocular and/or mucosal symptoms suggestive for SS, as well as to highlight correlations between histological findings and autoimmune profiles.
Material and methods
The study was conducted in accordance with the Declaration of Helsinki and was approved by the local Ethical Committee of the Azienda Ospedaliera Universitaria Senese (Siena, Italy): PROTOCOL RHELABUS 22271.
Inclusion criteria
We retrospectively evaluated data from patients who consecutively underwent MSGB in our department for suspected SS, in the period from March 2011 to December 2018. We included in the study all patients with a history of xerophthalmia and/or xerostomia and a positive/borderline result in the Schirmer test. The Schirmer test was defined as positive if ≤ 5 mm after 5 minutes and borderline if between 5 and 10 mm after 5 minutes.
Exclusion criteria
Exclusion criteria were the presence of a previous definite diagnosis of RA, systemic sclerosis, vasculitis, SLE, sarcoidosis, amyloidosis, idiopathic inflammatory myopathies, human immunodeficiency virus (HIV), hepatitis C virus (HCV), end-stage renal disease, graft-versus-host disease (GVHD), IgG4 related disease (IgG4-RD), amyloidosis, diabetes mellitus, lympho- or myelo-proliferative diseases and a history of head and neck radiotherapy.
Interventions
We performed the punch biopsy technique proposed by Guevara-Gutiérrez et al. [4], using a 4 mm punch and 4–0 absorbable sutures, following local anesthesia with lidocaine; salivary glands samples were subsequently fixed in formalin and evaluated by the pathology department of Siena using the focus score (FS) and Chisholm and Mason (CM) grading. The histological report was considered putative for SS when CM3 and/or FS ≥ 1. We excluded from the evaluation all invalid histological reports (i.e. total surface area obtained < 4 mm2).
Antinuclear antibodies (ANA) were detected by indirect immunofluorescence on Hep2 cells (Euroimmun, Lubeck, Germany), anti-extractable nuclear antigens (ENA) using both fluorescent enzyme immunoassay (FEIA-Thermo Fisher) and Western blot (WB, ANRIKA), while anti-double stranded DNA (dsDNA) antibodies and anti-citrullinated protein antibodies (ACPA) were detected with FEIA.
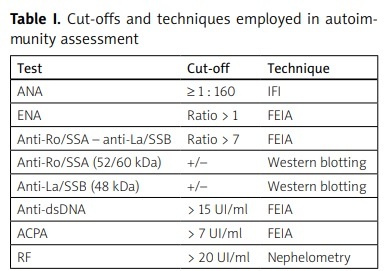
Rheumatoid factor (RF) IgG was concomitantly assessed. Antinuclear antibodies with speckled and SSA pattern were considered as SS-associated pattern (ANAsp). Table I shows in more detail cut-offs and techniques employed in laboratory assessment.
Table I
Cut-offs and techniques employed in autoimmunity assessment
Statistical analysis
Descriptive statistics included sample size, percentages, medians, and standard deviations. Univariate logistic regression analysis was used to identify autoimmune laboratory findings associated with diagnostic histological evidences, with CM ≥ 3, FS ≥ 1 and MSGB positivity (either CM ≥ 3 or FS ≥ 1) sequentially used as dependent variables and the following findings used as independent variables: ANA titer > 1 : 80, ANA patterns, ENA titer, anti-SSA/Ro positivity, anti-SSA/Ro titer, anti-SSB/La positivity, RF positivity, RF titer, ACPA positivity, ACPA titer, anti-DNA positivity, anti-DNA titer, western blotting positivity (identification of 48 kDa, 52 kDa or 60 kDa).
Autoimmune laboratory variables significantly associated with diagnostic histological findings in univariate analysis were later used as independent variables in multivariate stepwise logistic regression analysis, with CM ≥ 3, FS ≥ 1 and MSGB positivity remaining dependent variables.
Odds ratio (OR), its statistical significance and the corresponding 95% confidence interval (CI) were evaluated for independent variables significantly associated with dependent variables at regression analysis. The SPSS software, version 24, was used for all statistical computations, always considering a significance level of 95% (p-value < 0.05).
Results
A total of 1,266 patients (108 males, 1,158 females) were included in our study. The median age of patients at biopsy was 55.22 ±13.51 years (range: 15–87). Two biopsies were previously excluded due to the presence of amyloidosis in Congo red staining, and marginal zone B cell non-Hodgkin lymphoma, respectively.
Using CM, 614 out of 1,264 biopsies (48.57%) were considered consistent for SS (CM ≥ 3): in particular, CM = 0 was observed in 132 cases (10.4%), CM = 1 in 376 (29.8%), CM = 2 in 141 (11.2%), CM = 3 in 287 (22.7%), and CM = 4 in 327 (25.9%).
Focus score assessment, performed in 714 subjects, led to the following results: FS < 1 in 339 patients (47.3%), FS ≥ 1 but < 2 in 242 (33.9%); FS ≥ 2 but < 3 in 95 (13.3%); FS ≥ 3 but < 4 in 23 (2.9%); FS ≥ 4 in 16 (2.2%). Subjects’ ANA titers and patterns are summarized in Table II.
Table II
Antinuclear antibody titers and patterns
A positive histological evaluation was found in 274/442 (61,99%) patients whose ANA titer was > 1 : 80, and in 162/244 (66.39%) subjects with ANAsp. Anti-extractable nuclear antigens screening (FEIA) was positive (ratio > 1) in 263/963 patients (27.3%).
In particular, anti-SSA/Ro were positive in 186 patients, 129 of whom (69.35%) had histological findings consistent for SS. Among SSA/Ro negative patients, 61/111 (54.95%) had a positive biopsy.
Anti-SSB/La were positive in 49 patients; 44 of them (89.79%) had a positive biopsy. Notably, in our cohort, all patients with anti-SSB/La positivity (FEIA) were also positive for anti-SSA/Ro.
Anti-extractable nuclear antigens WB highlighted positivity of anti-SSA/Ro and anti-SSB/La in 139 and 27 patients, respectively; 73 of them (46.49%) reported CM ≥ 3 and/or FS ≥ 1. Thirty-eight patients were positive for both ANA and ENA, but negative for anti-SSA/Ro; 24 (63.15%) had a positive biopsy.
A total of 510 patients were negative for both ANA and ENA and 202 underwent ENA WB: 80 subjects (39.60%) were positive for anti-SSA/Ro (52 and 60 kDa) and/or anti-SSB/La (48 kDa) and 20 of them (36.25%) had a positive biopsy. One hundred patients were fully negative for ANA/ENA (FEIA) and ENA WB. Forty-three of them (43%) had histological findings suggestive for SS.
Rheumatoid factor was positive in 87 out of 256 tested patients (33.98%); 54 of them (62.06%) proved positive in histological examination. Anti-double stranded DNA was positive (> 15 IU/ml) in 6 patients, ACPA (> 7 IU/ml, FEIA method) in 38. In more detail, dsDNA was tested in 214 biopsy-positive subjects, being positive in 5 (2.3%). The ACPA were positive in 27 out of 190 (14.21%) patients with CM ≥ 3 and/or FS ≥ 1.
The percentages of histological positivity, according to the different immunologic profile, are summarized in Table III.
Table III
Histological positivity expressed according to different autoimmune profiles
In univariate binary logistic regression (Table IV), both CM ≥ 3 and FS ≥ 1 were significantly predicted by: ANA titer (OR = 1.317, p < 0.0001), ENA titer (OR = 1.055, p < 0.0001), anti-SSA/Ro positivity (1.758, p = 0.023), anti-SSA/Ro titer (OR = 1.005, p = 0.003), anti-SSB/La positivity (OR = 5.951, p < 0.0001), RF positivity (OR = 1.980, p = 0.004), ACPA positivity (OR = 2.826, p = 0.005). In multivariate analysis, CM ≥ 3 and MSGB positivity were significantly associated with ANA titer (OR = 1.404, p = 0.017 in both cases); FS ≥ 1 was not associated with laboratory findings.
Table IV
Univariate binary logistic regression for CM ≥ 3 and/or FS ≥ 1
Finally, 44 patients presenting with ANA ≥ 1 : 320 were positive at biopsy in 28 (63.3%) cases, 6 patients with RF positivity had a positive biopsy in 5 (83.33%) cases, while 6 patients presenting both with ANA ≥ 1 : 320 and RF positivity had a positive biopsy in 5 cases (83.33%) (Table V).
Discussion
Sjögren’s syndrome is a complex disease and should be considered as a multi-organ condition, since 46% of patients may report an extra-glandular involvement and up to 15% could experience severe systemic manifestations [5].
Arthralgias and arthritis are the most common features, while leucopenia, skin, lung, kidney and central or peripheral nervous system involvement are respectively less common. Salivary gland lymphoma, although quite uncommon, is the most feared long-term complication of SS and it is usually represented by mucosa-associated lymphoid tissue non-Hodgkin lymphoma, diffuse large B cell lymphoma and nodal marginal zone lymphoma.
Sjögren’s syndrome should be always considered in patients reporting dry mouth and/or eyes, but differential diagnosis of these symptoms can be quite challenging. The prevalence of xerophthalmia and/or xerostomia is notable, although not homogeneous [6–8], and is higher among elderly people [9] and women.
Thus, age-related sicca syndrome is probably the most common cause of xerostomia and/or xerophthalmia, followed by lifestyle factors (tobacco, caffeine, alcohol, snoring) and drug-induced sicca syndrome.
Other, less common causes of xerostomia and/or xerophthalmia are chronic viral infections, GVHD, sarcoidosis, Heerfordt syndrome (a rare manifestation of sarcoidosis), tuberculosis, Parkinson’s disease, hemochromatosis, end stage renal disease, amyloidosis, IgG4-related disease, viral infections, diabetes mellitus, autoimmune thyroid diseases, head and neck radiotherapy and granulomatosis with polyangiitis [10, 11].
All these conditions must be ruled out when suspecting SS and an accurate pharmacological anamnesis should be collected, since also a large number of drugs are capable of inducing sicca syndrome [12].
The 2016 ACR/EULAR classification criteria for primary SS [3] place the histopathological criterion in a pivotal position, beside specific autoimmunity (anti-Ro/SSA). The minor salivary gland biopsy is a fast, safe and low-cost procedure, that allows the removal and then the histological examination of the patient’s glandular tissue, being therefore the most used in clinical practice [13].
Autoimmunity evaluation stands beside histopathology as a cornerstone for SS diagnosis, since traditionally, this disease is characterized by positivity of autoantibodies against SSA/Ro and SSB/La, present in 50–70% of the patients [14].
The strong association between anti-SSA/Ro and positive histology is well known [15, 16] and is confirmed by our data, as about 70% of our anti-SSA/Ro-positive patients reported a biopsy consistent with SS.
On the other hand, 54% of anti-SSA/Ro-negative patients tested positive at histological evaluation, highlighting the importance of performing further research, such as MSGB, in autoimmunity-negative subjects with highly suggestive symptoms.
Recently, the presence of anti-SSB/La has been excluded from the classification criteria based on significant histological negativity found in anti-SSB-positive subjects in the validation cohort [3]. However, the data examined only took into account subjects who tested positive for anti-SSB/La antibodies but negative for anti-SSA/Ro antibodies.
In our cohort all anti-SSB/La-positive Ab patients were also positive for anti-SSA/Ro Ab, and 89.79% of these subjects reported a biopsy consistent with SS. From a practical point of view, the double positivity of anti-SSA/RoAb and anti-SSB/LaAb appears to be strongly associated with a positive histological evaluation, making MSGB de facto redundant in this subgroup of patients.
Antinuclear antibodies are positive in up to 80% of SS patients and in almost all subjects with an aggressive disease course [17]. In our study, we found ANA positivity in 442/1,007 patients (43.89%) and 274 of them (61.99%) tested positive in histological evaluation: the ratio was slightly higher considering only ANAsp (162/244, 66.39%), but lower than reported in the literature [18]. Such a difference could be at least partially explained by the higher cut-offs employed to assess ANA positivity (≥ 1 : 160 instead of ≥ 1 : 80).
In addition, patients who tested positive for ANA and ENA but negative for anti-SSA/Ro still had a positive biopsy in 63.15% of cases. In our opinion, these data leads again to methodological (mostly regarding the sensitivity of laboratory techniques used for autoimmunity detection) as well as immunological considerations such as the hypothesis that other antibodies may play a role in the pathogenesis of SS.
A positive biopsy was reported in 73/157 (46.49%) patients with positive anti-SSA/RoAb and/or anti-SSB/LaAb assessed using ENA WB. Twenty-five ENA-WB positive subjects were negative for both ANA and ENA. Notably, 25/70 (35.71%) ANA and ENA (FEIA) negative, ENA WB positive and 43/100 (43%) fully negative patients (ANA, ENA, and ENA WB) had a positive biopsy. The lack of statistically significant differences between these subgroups should make one reconsider the usefulness of WB ENA assessment in SS’s diagnostic process.
Rheumatoid factor positivity is reported in 40–50% of patients affected by SS [16, 19], often in association with anti-SSA/Ro and SSB/La; its prognostic role is uncertain, but it seems related to a more severe and systemic disease [20–22] as well as to the risk of developing a malignancy [23]. Our findings seem to confirm these data, as RF was found in 54 out of 87 subjects (62%) with histological findings consistent for SS, indicating its value in the diagnosis of SS.
Among the other autoantibodies reported in association with SS, ACPA are detected in 3–10% of patients, but their role in predicting erosive or non-erosive arthritis is still a matter of debate [24]. In our cohort, 190 biopsy-positive subjects were evaluated for ACPA and 27 of them (14.21%) tested positive, probably due to the presence of a certain number of subjects who would later develop an overlap syndrome.
Many other antibodies, such as anti-dsDNA and myositis-specific antibodies, have been described in association with SS, but their prevalence is low and their clinical role unknown [25]. Such findings are confirmed in our cohort, in whom only 5 out of 214 biopsy-positive patients (2.3%) were also positive for anti-dsDNA.
Univariate logistic regression confirmed that a positive biopsy is significantly predicted by ANA titer, ENA titer, anti-SSA/Ro positivity and titer, anti-SSB/La, RF and ACPA positivity. These findings demonstrate the importance of a complete autoimmune evaluation before performing MSGB, and confirm the redundancy of this procedure in those subjects with both symptoms and autoimmunity consistent with SS.
In uncertain cases, with no specific antibodies (e.g. ANA/ENA+, anti-SSA/Ro–), the decision to perform an MSGB should be made by the clinician, considering symptoms, age, comorbidities and global autoimmunity, including RF. Concerning this, in our cohort only a few patients were negative for anti-SSA/Ro but positive for RF and/or ANA ≥ 1 : 320 (Table V).
Nevertheless, a notable percentage of them had a positive biopsy. These findings could bring clinicians to reconsider the role of non-specific autoimmunity, despite its recent exclusion in the 2016 classification criteria.
Study limitations
The main limitation of our study was the lack of clinical data and therefore the impossibility to correlate histological and laboratory data with course of the disease in particular patient, as well as with the potentially onset of lymphomas and systemic (organ) involvement.
Conclusions
Our findings corroborate the prominent role of auto-immunity evaluation in the diagnosis of SS, pointing out the valuable contribution made by both specific and non-specific autoantibodies.
At the same time, MSGB is confirmed as an essential tool for SS diagnosis, especially in the group of patients with autoimmune (immunological)-negative tests but with symptoms highly suggestive for SS.