Introduction
Chronic plaque psoriasis (CPP) is a chronic inflammatory dermatological disease. The CCP is characterized by erythematous plaques, dense silvery scales, and red/white skin blotches [1]. The association between psoriasis-induced chronic inflammation and the development of cardiovascular and metabolic diseases has repeatedly been documented. Non-alcoholic fatty liver disease (NAFLD) is one of the highly prevalent metabolic diseases/complications in patients with psoriasis, especially obese ones [2, 3]. The estimated percentage of NAFLD in patients with psoriasis is 85% [4, 5] but the cause of this high percentage is not fully explained [6].
Psoriasis-induced chronic inflammation (evoked from skin- or blood-derived cytokines) [7], increased energy intake [8], and lack of energy expenditure [9] are the suggested causes of development of NAFLD and other cardiovascular risk factors (e.g. metabolic syndrome) in psoriatic patients [7].
Recently, weight loss has been a highly recommended intervention in many disorders [10]. It is recommended in psoriatic patients to improve the severity of psoriatic symptoms [11], psoriasis-induced chronic systemic inflammation, psoriasis-associated cardiovascular risk factors [12], quality of life, and the efficacy of anti-psoriatic drugs, especially in mild-to-severe stages of CPP [13].
No study has investigated the effect of weight loss on triglycerides, liver enzymes, psoriasis severity, and quality of life in men with psoriasis and NAFLD, so this study was the first to examine this effect.
Material and methods
Inclusion criteria
The men with psoriasis were recruited through fixed posters on the walls of the internal diseases and dermatology outpatient clinics of Zefta and Mitghamer General Hospitals. This study included 60 class I obese men. The men were stable mild-to-severe psoriatics with NAFLD (the diagnosis of fatty liver was confirmed through abdominal ultrasonography). The included men were ≥ 18 years old. The recruited psoriatic men received immunosuppressive therapies without changes in the last 6 months.
Exclusion criteria
Men with other forms of psoriasis, such as arthropathic/guttate, pustular, and erythrodermic psoriasis, were excluded. Men with psoriasis who had received phototherapy and biologic interventions for at least 12 weeks before enrolment were also excluded.
Psoriatic men with cancer, hepatitis, other autoimmune diseases, or disorders within the kidneys or the heart were excluded. Psoriatic smokers and alcoholics were also excluded. Psoriatic men using weight loss prescriptions or following diet and/or exercise programs were also excluded.
Randomization
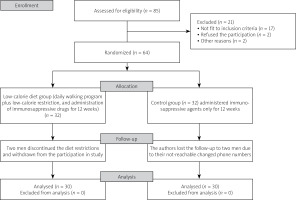
The included psoriatic men were randomly assigned via a computerized random block two-group list to one of the following psoriasis groups: the low-calorie-diet group (30 men received immunosuppressive drugs, followed a low-calorie diet, and increased their energy expenditure through a daily 15,000-step outdoor walking program for 12 weeks) or the control group (30 men received immunosuppressive drugs only) (Fig. 1).
Low-calorie diet
The low-calorie-diet meal plan was formulated based on the total energy intake or basal metabolic rate (BMR) of participating men. The basal metabolic rate – calculated from the Harris-Benedict equation which was revised by Mifflin and St Jeor in 1990 – was obtained as follows: BMR of men = (10 × weight in kilograms) + (6.25 × height in centimeters) – (5 × age in years) + 5 [14]. After calculating the total energy intake, the third author (who supervised the nutrition program of this study) subtracted 500 kcal/day as a 500 kcal/day deficit.
Based on the recommended intake of nutrients, a well-balanced low-calorie-diet meal plan (3 main meals per day) was introduced to each psoriatic man. Meal-plan macronutrients contained the following items: fats (20–30%), proteins (10–15%), carbohydrates (55–65%), dietary fiber, as well as fruits/vegetables [15]. Men were interviewed every week to follow, discuss, and monitor their adherence to the suggested low-calorie-diet meal plan.
Walking program
Psoriatic men were recommended to achieve a goal-setting of 15,000-step outdoor walking per day. This walking program was conducted at a moderate intensity (60% of target heart rate, THR). Target heart rate was calculated from the Karvonen formula (THR = HRrest + (HRmax– HRrest) × training fraction), while HRmax was calculated from the following equation: 220 – age (years) and the traction fraction was 60% HRmax [16, 17]. The heart rate and the number of steps were recorded/monitored through a bracelet digital pedometer during the outdoor walking program.
As a way to confirm adherence to the recommended program of physical activity, a daily phone call to each participant was made to complete the recommended daily outdoor walking program.
Outcomes
The primary outcome of this study was the Psoriasis Area and Severity Index (PASI). A licensed dermatologist used the validated PASI scoring system, and assessed erythema condition, scaliness state, and density of psoriatic plaques in different body regions (head, neck, upper and low limbs, and trunk). Scores of PASI ranged from 0 to 72 (higher scores indicated severe psoriatic conditions) [18].
The secondary outcomes of this study were anthropometry (weight, body mass index – BMI, and waist circumference – WC), triglycerides (TG), liver enzymes (alanine transaminase – ALT, and aspartate transaminase – AST), and Dermatology Life Quality Index (DLQI).
The DLQI is a 10-item self-answered questionnaire that was designed to assess the quality of life in patients with chronic skin diseases [19]. The DLQI assesses the effect of skin diseases, including psoriasis, on daily activities, symptoms and sentiments, leisure, school or work, personal contacts or relationships, and treatment. The DLQI is obtained by summing the score of each item resulting in a maximum of 30 and a minimum of zero. The higher the DLQI score, the more quality of life is impaired [20].
Statistical analysis
The normal distribution of psoriatic men’s data was confirmed through the Shapiro test. Paired and unpaired tests of IBM SPSS (version 18) compared the men’s data within and between groups, respectively.
Power analysis
Before starting the psoriatic interventions in both men’s groups, the sample size was calculated using Franz-Fual G*power (3.1.9.2, Germany). Based on men’s data from a pilot study, the effect size of the main outcome (PASI) was 0.82. Taking into account a 20% dropout rate, the proper minimum sample size for this psoriatic study was 32 men in each men’s group with a power of 90%.
Bioethical standards
The current randomized-controlled psoriatic trial (NCT05125757) was conducted following the Declaration of Helsinki. The consent form and psoriatic protocol were reviewed by the Local Cairo-University Scientific Board (the Permission Ref. No. of the Physical Therapy Faculty was P.T.REC/012/003344). According to the guidelines of Consolidated Standards Of Reporting Trials (CONSORT) the checklist was completed and all steps were implemented.
Results
Before starting the psoriatic interventions, there was no significant difference between the weight-loss and control groups in terms of age, immunosuppressive therapies, and fasting blood glucose (Table I). The before-treatment values of the primary outcome (PASI) and secondary outcomes (AST, BMI, ALT, WC, TG, and DLQI) did not show a significant difference between the weight-loss and control groups (Table II).
Table I
Basic data of psoriatic groups
Table II
Mean ±SD outcomes of the trial
| Outcomes | Weight-loss group | Control group | p-value (among fatty liver groups with psoriasis) |
|---|---|---|---|
| Pre BMI [kg/m2] | 31.99 ±3.92 | 32.60 ±4.09 | 0.557 |
| Post 12-wk BMI [kg/m2] | 29.98 ±3.56 | 32.83 ±4.10 | 0.005* |
| Within-group p-value | < 0.001* | 0.053 | |
| Pre WC [cm] | 109.78 ±13.11 | 108.86 ±13.13 | 0.786 |
| Post 12-wk WC [cm] | 103.13 ±10.54 | 109.63 ±13.69 | 0.043* |
| Within-group p-value | < 0.001* | 0.056 | |
| Pre AST [U/l] | 32 ±14.61 | 30.06 ±14.83 | 0.611 |
| Post 12-wk AST [U/l] | 21.46 ±8.34 | 30.46 ±14.81 | 0.005* |
| Within-group p-value | < 0.001* | 0.103 | |
| Pre ALT [U/l] | 37.40 ±16.46 | 36.90 ±15.04 | 0.902 |
| Post 12-wk ALT [U/l] | 21.30 ±8.06 | 37.36 ±15.23 | 0.0001* |
| Within-group p-value | < 0.001* | 0.055 | |
| Pre TG [mg/dl] | 165.16 ±90.82 | 166.43 ±88.10 | 0.956 |
| Post 12-wk TG [mg/dl] | 124.20 ±48.54 | 179.56 ±89.91 | 0.004* |
| Within-group p-value | < 0.001* | 0.167 | |
| Pre PASI | 8.16 ±5.94 | 9.80 ±7.76 | 0.361 |
| Post 12-wk PASI | 6.05 ±4.77 | 8.71 ±6.82 | 0.08 |
| Within-group p-value | < 0.001* | 0.052 | |
| Pre DLQI | 20.26 ±5.67 | 19.16 ±4.34 | 0.402 |
| Post 12-wk DLQI | 12.93 ±5.32 | 17.36 ±4.90 | 0.001* |
| Within-group p-value | < 0.001* | 0.078 |
While the pre-versus-post comparison of primary and secondary outcomes of the control group (received immunosuppressive therapies only) did not show a significant improvement (p > 0.05), the same comparison within the weight-loss group showed a highly significant improvement in primary and secondary outcomes (p < 0.001). While the between-group post-treatment comparison of secondary outcomes demonstrated a significant improvement in favor of the weight-loss group, the primary outcome (PASI) did not show the same result (Table II).
Discussion
This study is the first one reporting a significant improvement in WC, PASI, BMI, ALT, DLQI, AST, and TG after a 12-week low-calorie-diet program in class I obese men with CPP and NAFLD. The improvement mechanism of psoriasis severity after the applied weight-loss program is not fully clarified.
Dietary interventions are associated with a decreased body mass, lowered visceral adipose-tissue size [21], improved skin-derived inflammatory background of psoriasis, improved adipocyte-induced systemic chronic inflammation, decreased size of hypertrophic adipocytes [22], and lowered hepatic lipid buildup and liver enzymes in obese patients with CCP [23].
Exacerbations of psoriasis are thought to be influenced by psychological stressors. Exercise-induced strength of the immune system is associated with improved psychological stress, mood, depression, sense of wellbeing, quality of life, and exacerbations of psoriasis [24].
A study conducted in 2014 supported our results. This study investigated the response of PASI after adding a 20-week weight-loss program (diet restriction with 120 minutes of exercise per week) to the systemic pharmacotherapies of active psoriasis in overweight and obese patients. Patients (n = 151) who underwent the supervised weight-loss program showed a 48% reduction in the median PASI. Patients (n = 152) who received general information about diet restriction and increasing physical activity showed a 25.5% reduction in the median PASI [25].
Supporting the present results, besides the significant improvements in TG, DLQI, WC, and BMI, a study concluded that pharmacological agents and 12-week energy-intake restriction showed more PASI improvement compared to pharmacological agents alone in obese patients with psoriasis [20].
In support of the present findings, besides the improvements in insulin, liver enzymes (ALT and AST), and inflammatory markers (C-reactive protein, monocyte chemoattractant protein-1, leptin, and adiponectin), obese women with CPP who received cyclosporine and 24-week energy-intake restriction showed more PASI improvement than women who received cyclosporine only [26].
Again, patients with psoriasis who received biological treatment and a 24-week low-calorie diet showed more PASI improvement than patients who received biological treatment only [27]. Additionally, besides the improvement in PASI, AST, DLQI, ALT, and TG, the strict adherence to 24-week energy-intake restriction increased the efficacy of topical treatments in ten obese patients with CPP [23].
Furthermore, supporting our findings, weight loss via a 16-week low-calorie diet is highly associated with significant PASI and TG improvements in class I obese patients with CPP [28]. Additionally, in support of our results, the randomized assignment of overweight patients with psoriasis to a low-calorie-diet group (n = 16) or control group (n = 16, patients were advised to continue eating normal healthy foods) produced greater improvements in BMI, WC, PASI, and DLQI in the low-calorie-diet group [29].
Based on the results of a published systematic review in 2015, as support to our results, weight loss through energy-restriction-intake programs guarantees a greater response of psoriasis to systemic or immunosuppressive pharmacotherapies [30]. The significant loss of body weight in our study was supported by another study that reported that obese patients with psoriasis who were in the disease-remission period and following a low-calorie diet showed a significant body mass decrease after 12 weeks [31]. Again, supporting us, the nonconventional weight-loss procedures such as jejunoileal [32] or gastric bypass bariatric surgery guarantee a greater effect of systemic agents on psoriasis severity in obese patients [33, 34].
Future research with longer tracking periods will be helpful to verify whether the improvement trend involving the PASI, DLQI, TG, AST, and ALT has been sustained. It will also be beneficial for future psoriasis trials to compare the effect of different diet protocols on psoriasis severity and psoriasis-induced NAFLD. Lastly, it will also be beneficial for future trials to compare the effect of dietary interventions versus supervised exercise rehabilitation programs on the measured outcomes.
It is known that the inflammatory processes caused by metabolic syndrome overlap with the inflammatory processes caused by psoriasis. Therefore, proving this relationship seems interesting and should lead to further clinical studies using similar assumptions. It is also worth comparing the results of groups treated with lifestyle changes (diet plus exercise) and different drugs that were administered in this study, of course on a much larger number of patients.
Study limitations
The authors analyzed the effect of the weight-loss program on psoriasis severity in class-I obese men with CPP and NAFLD, but this effect was not investigated in class II or class III obese patients since obesity and NAFLD rates have increased alarmingly among CPP patients. The psoriatic study has another limitation, because the sample is limited to men. This work could benefit from including women to determine the comparative responses of psoriatic women and men to weight loss programs.
However, this study also has significant strengths. The patients who completed this weight-loss interventional trial after the low dropout comprised 60 psoriatic men. To carry out a better assessment after the investigation, all of the men were at a similar baseline. To increase the adherence of men to the low-calorie-diet meal plan, the dietary intervention was reviewed weekly in face-to-face consultations as a support to the applied weight loss program.