Introduction
Psoriatic arthritis (PsA) was classified as spondyloarthritides in the 1970s because of its resemblance to ankylosing spondylitis and reactive arthritis [1, 2].
Psoriatic arthritis is an inflammatory disease that affects skin and nails, as well as causing peripheral and axial joints, enthesitis, and dactylitis [3]. Skin psoriasis manifests ten years before joint inflammation in 85% of cases [4]. Thus, dermatologists are usually the first physicians who encounter the development of the PsA’s initial manifestations. The early diagnosis and treatment help to avoid further joint involvement and disabilities. There is a problem in the early diagnosis of PsA worldwide, which may be attributed to the dermatologists missing PsA symptoms and signs and a lack of effective screening tools. To know the risk of PsA development, a close collaboration between rheumatologists and dermatologists with expertise in psoriatic disease is needed [5].
This issue is more pronounced in developing countries, particularly those in the Middle East and Africa. A group of experts recommends starting multidisciplinary clinics involving both specialties (Rheumatology and Dermatology) for early diagnosis, treatment, and management of PsA in Africa and the Middle East countries [6].
This study aims to establish the prevalence of PsA in psoriasis patients attending the dermatology clinic, identify the problem of underdiagnosis of PsA, and determine the clinical predictors of PsA development.
Material and methods
The authors conducted a cross-sectional observational study on 200 patients diagnosed with psoriasis (Ps) who visited the dermatology outpatient clinic Kasr Alainy Hospital, Cairo University. The data were collected from all patients who attended the dermatology clinic between December 2016 and December 2017 including a pediatric group. The studied group included in total 200 psoriasis patients, 51% (n = 102) female and 49% (n = 98) male. There were 22 pediatric psoriasis patients, who made up 11% of total enrolled patients; among them 59% were female (n = 13) and 41% were male (n = 9).
The age range for all participants was 5–75 years, with a median of 44 years, and the psoriasis disease duration ranged between 0.08 and 45 years, with a median of 7 years. Psoriasis was diagnosed between the ages of 0.2 and 74 years, with a median of 31 years.
All procedures performed in this study followed the 1964 Helsinki Declaration and its later amendments.
The approval of the Bioethics Committee at the University of Cairo was obtained and all adult patients enrolled in the study agreed to participate and provided written consent. Informed consent on examining the pediatric patients was obtained from parents or guardians in accordance with legal standards.
Assessment of psoriasis patients
History taking and examination were done, including site, type, and extent of psoriasis. For estimating the body surface area (BSA) of psoriatic lesions, the rule of nines was used: 9% coverage for the head and neck, 9% for each arm, 9% for the anterior and posterior legs, and 9% for each of the 4 trunk quadrants, leaving 1% for the genitalia [7].
The severity of psoriasis was determined by employing BSA, using the following scoring: < 3% mild case of psoriasis, 3–10% moderate case of psoriasis, and > 10% severe case of psoriasis [8]. The psoriasis area and severity index (PASI) combines the severity and percentage of the affected area into a single score ranging from 0 (i.e., no disease) to 72 (i.e., maximal disease) [9].
The psoriasis disability index (PDI) was calculated for assessing functional lifestyle disabilities. The psoriasis disability index is a 15-item scale. The item scores are summed to a total score with a range of 0–45 [10].
Screening all psoriasis patients for psoriatic arthritis
All psoriasis patients were screened for PsA using the following methods:
the Psoriasis Epidemiology Screening Tool (PEST) is composed of 5 questions and a drawing of a mannequin, Yes and No answers take scores of 1 and 0, respectively, and if the total score ≥ 3, it is positive [11],
the Early Arthritis for Psoriatic Patients (EARP) questionnaire consists of 10 questions, Yes and No answers receive scores of 1 and 0, respectively, while a score of ≥ 3 indicates a positive response [12],
the patients with a positive PEST and/or EARP result were assessed for fulfilling the classification for psoriatic arthritis (CASPAR) criteria, indicating inflammation in the joints, spine, or entheses with ≥ 3 points from 5 categories [13].
Clinical assessment of patients with psoriatic arthritis
Full history and examination were performed for all PsA patients.
Assessment of comorbidities in psoriasis and psoriatic arthritis patients
Assessment of comorbidities in psoriasis and PsA patients through:
medical history of diabetes mellitus, hypertension, cardiac diseases such as ischemic heart disease (IHD), chest disease such as chronic obstructive pulmonary disease (COPD), malignancy, and hepatitis C virus (HCV) infection was taken from all patients,
examination: calculating body mass index (BMI) and waist circumference measurement were done for all patients,
blood tests were done for all patients including: serum uric acid, total cholesterol, triglyceride, low-density lipoprotein, high-density lipoprotein, fasting, 2 hour postprandial, and HCV antibodies by enzyme-linked immunosorbent assay.
Statistical methods
Variables such as age, gender, duration of psoriasis, age of psoriasis onset, psoriasis types, sites, psoriasis severity, and obesity were analyzed in multivariate analysis to detect predictors of PsA in patients with psoriasis.
Data were coded and entered using SPSS version 25. Data were summarized using mean, standard deviation, median, minimum and maximum in quantitative data. The frequency (number) and relative frequency (percentage) were calculated for categorical data. Comparisons between quantitative variables were performed using the non-parametric Mann-Whitney U test [14, 15]. The chi-square (c2) test was used to compare categorical data. The exact test was performed instead when the expected frequency was less than 5 [16, 17]. If the p-value ≤ 0.05, it is deemed significant.
Results
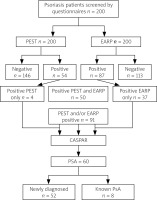
The total number of patients with a positive PEST questionnaire (score ≥ 3), suggestive of PsA, was 54, while the total number of patients with a positive EARP questionnaire (score ≥ 3), suggestive of PsA, was 87 (Fig. 1). There were 50 patients with both EARP and PEST positive. There were 91 patients who were positive for PEST and/or EARP, and they were assessed for the fulfilment of CASPAR criteria; 60 patients were classified as having PsA. Psoriatic arthritis was found in 60 patients (30%) with psoriasis; 8/60 patients (13.4%) were previously diagnosed with PsA.
Fig. 1
Study flow chart of 200 screened psoriasis patients.
CASPAR – classification for psoriatic arthritis, EARP – Early Arthritis for Psoriatic Patients Questionnaire, PEST – Psoriasis Epidemiology Screening Tool, PsA – psoriatic arthritis.
The demographic and clinical characteristics of 60 PsA patients are summarized in Table I. The following are the comorbidities found in patients with arthritis in descending order: dyslipidemia 39 (65%), obesity 34 (56.7%), metabolic syndrome 29 (48.5%), hypertension 20 (33.3%), diabetes mellitus 15 (25%), smoking 15 (25%), IHD 9 (15%), HCV infection 6 (10%), COPD 4 (6.7%), malignancy 2 (3.3%) patients; one had lymphoma and the other had rhabdomyosarcoma.
Table I
Demographic, clinical characteristics of psoriatic arthritis patients
Table II compares the demographics, clinical features, extent, psoriasis severity by PASI, and quality of life by PDI of PsA patients with non-PsA patients. It was found that patients with PsA had statistically significantly longer disease duration (p = 0.002) and lower frequency of classic plaque (p = 0.008). However, patients with PsA had statistically significantly higher frequency of nail psoriasis (p < 0.001) and erythrodermic psoriasis (p = 0.018) types. Regarding the site of psoriasis, PsA had a statistically significantly higher frequency of involvement of the knees (p = 0.008), genitalia (p < 0.001), flexure (p = 0.033), and intergluteal cleft (p < 0.001).
Table II
Comparison of demographic and clinical characteristics between psoriatic arthritis and non-psoriatic arthritis patients
| Parameters | PsA (n = 60) | Non-PsA (n = 140) | p-value | ||
|---|---|---|---|---|---|
| Age [years] (mean ±SD) | 45 ±10.79 | 40.70 ±19.5 | 0.07 | ||
| Gender | |||||
| Female | 36 | 40% | 66 | 60% | 0.10 |
| Male | 24 | 60% | 74 | 40% | |
| Psoriasis duration (median; range) | 10 | 0.08–45 | 6 | 0.08–44 | 0.002* |
| Type | |||||
| Classic plaque | 43 | 71.7% | 122 | 87.1% | 0.008* |
| Scalp psoriasis | 39 | 65% | 76 | 54.3% | 0.160 |
| Nail | 43 | 71.7% | 43 | 30.7% | < 0.001* |
| Guttate | 3 | 5.0% | 16 | 11.4% | 0.155 |
| Inverse | 7 | 11.7% | 20 | 14.3% | 0.619 |
| Erythrodermic | 11 | 18.3% | 10 | 7.1% | 0.018* |
| Pustular | 2 | 3.3% | 5 | 3.6% | 1 |
| Chronic palmoplantar pustulosis | 1 | 1.7% | 1 | 0.7% | 0.511 |
| Palmoplantar | 3 | 5% | 17 | 12.1% | 0.123 |
| Site | |||||
| Scalp | 48 | 80% | 95 | 67.9% | 0.081 |
| Face | 13 | 21.7% | 25 | 17.9% | 0.529 |
| Neck | 14 | 23.3% | 30 | 21.4% | 0.766 |
| Trunk | 36 | 60% | 80 | 57.1% | 0.708 |
| UL | 47 | 78.3% | 106 | 75.7% | 0.689 |
| LL | 46 | 76.7% | 107 | 76.4% | 0.971 |
| Palms | 15 | 25% | 28 | 20.0% | 0.430 |
| Soles | 13 | 21.7% | 26 | 18.6% | 0.613 |
| Elbows | 32 | 53.3% | 67 | 47.9% | 0.478 |
| Knees | 40 | 66.7% | 69 | 49.3% | 0.024* |
| Genitalia | 23 | 38.3% | 21 | 15% | < 0.001* |
| Flexures | 21 | 35% | 29 | 20.7% | 0.033* |
| Intergluteal cleft | 32 | 53.3% | 24 | 17.1% | < 0.001* |
| Extent [%] (median; range) | 17.5 | 0.2–97 | 16 | 0.1–95 | 0.602 |
| PASI (median; range) | 6.05 | 0.2–49 | 8.1 | 10–54 | 0.417 |
| PDI (median; range) | 13 | 0–34 | 9 | 0–53 | 0.024* |
The prevalence of comorbidities (hypertension, diabetes mellitus, obesity, ischemic heart disease, COPD, HCV infection, and malignancy) was compared between patients with PsA (60) and without PsA (140). As shown in Table III, patients with PsA had a significantly increased incidence of diabetes mellitus (p = 0.039), COPD (p = 0.029), obesity (p = 0.04), and metabolic syndrome (p = 0.004).
Table III
Comparison of comorbidities between patients with psoriatic arthritis and non-psoriatic arthritis
| Parameters | PsA (n = 60) | Non-PsA (n = 140) | p-value | ||
|---|---|---|---|---|---|
| Count | % | Count | % | ||
| Hypertension | 20 | 33.3 | 33 | 23.6 | 0.152 |
| Diabetes mellitus | 15 | 25.0 | 18 | 12.9 | 0.039* |
| COPD | 4 | 6.7 | 1 | 0.7 | 0.029* |
| IHD | 9 | 15.0 | 9 | 6.4 | 0.052 |
| Obesity | 34 | 56.7 | 56 | 40.0 | 0.043* |
| Dyslipidemia | 39 | 65 | 39/73 | 53.4 | 0.057 |
| Metabolic syndrome | 29 | 48.3 | 21/83 | 25.3 | 0.004* |
| Malignancy | 1 | 1.7 | 1 | 0.7 | 0.511 |
| HCV | 6 | 10.0 | 13 | 9.3 | 0.875 |
| Smoking | 15 | 25.0 | 41 | 29.3 | 0.536 |
Psoriatic arthritis predictors
Obesity (OR 7.0, 95% CI: 2.61–18.85), nail psoriasis (OR 5.02, 95% CI: 2.02–12.476), and intergluteal cleft site with psoriatic changes (OR 12.659, 95% CI: 4.302–37.255) were associated with an elevated risk of PsA. However, classic plaque psoriasis (OR 0.149, 95% CI: 0.051–0.433) and flexure site (OR 0.238, 95% CI: 0.076–0.746) were linked with decreased risk of PsA (Table IV).
Table IV
Logistic regression of independent predictors of psoriatic arthritis
| Parameters | p-value | OR | 95% CI | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Arthritis (dependent) | Classic plaque psoriasis | < 0.001* | 0.149 | 0.051 | 0.433 |
| Nail psoriasis | 0.001* | 5.020 | 2.020 | 12.476 | |
| Flexures | 0.014* | 0.238 | 0.076 | 0.746 | |
| Intergluteal cleft | < 0.001* | 12.659 | 4.302 | 37.255 | |
| Obesity | < 0.001* | 7.024 | 2.617 | 18.854 | |
Discussion
In this study, the prevalence of PsA was 30%, with a mean age of 45.48 ±10.79 years. Further, psoriasis preceded the onset of PsA in 46 (76.6%) patients, arthritis occurred before psoriasis in 6 (10%) patients, and both psoriasis and arthritis occurred simultaneously in 8 (13.3%) patients.
According to a recent meta-analysis, the prevalence of PsA in the general population is about 0.13% [18]. Another meta-analysis of the prevalence of PsA among skin psoriasis patients included 266 studies and 976,408 patients with psoriasis. The pooled percentage of PsA was 19.7%. On the other hand, a study using the CASPAR found a prevalence of PsA of 23.8% [19]. The present results showed a 30% prevalence of PsA.
This finding was consistent with a previous Egyptian study [20], although different criteria for diagnosis were used. In that study, 100 psoriasis patients were examined for PsA, which was diagnosed by the presence of joint symptoms, absence of rheumatoid nodules, negative rheumatoid factor, and any radiological manifestations. It was found that 32.0% of the patients had PsA. An earlier Egyptian study [21] was conducted on 276 psoriasis patients who attended the dermatology clinic for a year. They were examined for the presence of clinical arthritis, but no arthritis was found. A previous study gathered 82 psoriasis patients over a 6-month period from 3 dermatology centers in El Minia, Egypt [22]. The study screened patients with PsA using unique sets of criteria. The patients were considered to have PsA if they had clinical or radiological arthritis, spondylitis, enthesopathy, dactyl, or osteoperiostitis. According to these sets of criteria, they found a higher prevalence of PsA, with a rate of 72.8% (59 patients).
In comparison with different regions worldwide, our results were compatible with several European studies; for example, one study [23] found a 30% prevalence of PsA in 949 psoriasis patients from Europe and South America. Moreover, Henes et al. [24] documented a 30% prevalence of PsA in 404 patients with psoriasis in Germany (multicenter study). In a similar Turkish hospital-based study, 32 patients (25.4%) out of 126 psoriasis patients had PsA [25]. Moreover, Ranza et al. [26] reported a prevalence of 33% of PsA in a large number of Brazilian psoriasis patients.
Regarding the studies in Asian countries, we found lower prevalence ranging from 1% [27] to 11% [28] among psoriasis patients. This variation may be due to specific geographical characteristics of the disease and genetic differences.
A 12-study systematic review found that the prevalence of undiagnosed PsA among psoriasis patients ranged from 4.2 to 33.6%. In the meta-analysis of all 12 studies, the estimated prevalence of PsA was 15.5% [29]. There have been studies with very similar results to the current one; for instance, research in Ireland found that 29% of psoriasis patients followed up at dermatological clinics had undiagnosed PsA [30], another study [12] in Italy reported a frequency of 31%, and a study [31] in America found a prevalence of 33%.
This prevalence result suggests that psoriasis patients should be completely assessed for PsA presence and highlights the importance of screening tests in dermatology practice, reflecting the importance of the multidisciplinary clinic (Dermatology-Rheumatology clinic). However, the logistics of this clinic and the unavailability of concerned dermatologists, nurses, and clinic organizers are still restrictive factors in the Middle East and Africa [6].
Comparing psoriasis patients with and without PsA in univariate analysis, it was found that patients with PsA had statistically significantly longer disease duration (p = 0.002). This finding corroborated a recent population-based study, revealing that PsA incidence was associated with a longer disease duration of psoriasis (p = 0.0001) [32]. Patients with PsA showed a highly significant reduction in the frequency of classic plaque (p = 0.008).
The following was the frequency of comorbidities found in patients with arthritis in descending order: dyslipidemia 39 (65%), obesity 34 (56.7%), metabolic syndrome 29 (48.5%), hypertension 20 (33.3%), diabetes mellitus 15 (25%), smoking 15 (25%) IHD 9 (15%), HCV 6 (10%), COPD 4 (6.7%) and malignancy 2 (3.3%) (one had lymphoma and the one patient had rhabdomyosarcoma). We found that patients with PsA had statistical significant higher incidence of diabetes mellitus (p = 0.039), obesity (p = 0.04), metabolic syndrome (p = 0.004), and COPD (p = 0.029).
Husted et al. [33] documented a high prevalence of hypertension, dyslipidemia, diabetes mellitus, and CVD in PsA compared to non-PsA patients [33]. A larger number of PsA (n = 1952) had more hypertension compared to psoriasis patients (over 20,000) [34].
It is widely recognized that metabolic syndrome is more prevalent in PsA than in other rheumatic disorders. However, a few research has been conducted to determine its prevalence compared to psoriasis without PsA [35]. We found that the incidence of metabolic syndrome is statistically significant higher in PsA than in non-PsA psoriasis patients. Therefore, the presence of arthritis might have a cause-effect relationship with the metabolic status.
The current results are consistent with those of Wilson et al. [36], demonstrating that scalp lesions, nail changes, and intergluteal/perianal lesions were significantly associated with a higher risk of arthritis. The risk of arthritis was 3.89-fold higher among patients with scalp psoriasis (HR 3.89, 95% CI: 2.18–6.94) than those without scalp lesions. In addition, psoriasis patients with nail dystrophy were threefold more likely to develop arthritis (HR 2.93, 95% CI: 1.68–5.12) than patients without nail dystrophy. Psoriasis patients with intergluteal/perianal lesions (HR 2.35, 95% CI: 1.32–4.19) had a 2.35-fold increased incidence of PsA compared to patients without these skin lesions.
Haroon et al. [30] performed a study on 100 consecutive psoriasis patients attending the dermatology clinic. The patients were assessed by 3 questionnaires and subsequently diagnosed with PsA using CASPAR criteria. A prevalence of 29% of newly diagnosed PsA among psoriasis patients was found. The clinical and demographic characteristics were investigated by univariate and multivariate analysis to detect predictors of PsA. The analysis showed that newly diagnosed PsA patients had more aggressive skin involvement as evaluated by PASI. There was no significant difference regarding age, sex, drugs used for treating psoriasis, duration of psoriasis, scalp, and nail involvement.
Ranza et al. [26] reported that when psoriasis patients with and without arthritis were compared on demographic and clinical parameters (psoriasis types and PASI), PsA incidence in psoriasis patients was mostly associated with older age and nail involvement. Interestingly, a multivariate regression model applied to those variables showed that male gender was a protective factor for PsA (OR 0.416, 95% CI: 0.201–0.861, p = 0.018), with no other predictors.
The present findings suggest that obesity is a predictor of PsA, which is consistent with a general population study conducted in the UK, reporting that obesity is accompanied by a higher risk of PsA and emphasizing the importance of weight control among psoriasis patients who have metabolic syndrome and obesity. The higher the BMI is, the greater is the prevalence of PsA in psoriasis patients. Compared with psoriasis patients with BMI < 25 kg/m2, the relative risk values for developing PsA were 1.09 (0.93–1.28) for BMIs of 25.0–29.9, 1.22 (1.02–1.47) for BMIs of 30.0–34.9, and 1.48 (1.20–1.81) for BMIs ≥ 35 [37].
A population-based study in the USA showed a categorized positive association between weight change in adults, measures of central obesity, and risk of PsA (p < 0.001). The analysis of psoriasis development during follow-up demonstrated a close association (p < 0.01), indicating that a higher risk of PsA is linked with obesity among psoriasis patients [38].
Concerning nail lesions as a predictor of PsA, of all the clinical data examined so far to predict PsA, the most powerfully associated has certainly been nail disease. In a recently published study by Gisondi [39] the findings supported PsA nail involvement as a predictor of PsA development. An earlier study that intended to identify the clinical impact of nail involvement in 661 psoriasis patients showed an association between nail dystrophy and PsA, with an OR of 3.25 (95% CI: 2.16–4.90) [40].
Concerning medications used in psoriasis treatment and their impact on PsA development, it was found that treating patients with psoriasis with biologics might reduce the risk of developing PsA [41].
The main limitation of the present study was the small sample size, where we tried to recruit all psoriasis patients followed up in dermatology clinics during one year, but we could not.
Conclusions
Thirty percent of the patients with psoriasis had PsA, and among them 86.6% were undiagnosed, reflecting a high percentage of undiagnosed PsA that missed the early diagnosis in the dermatology clinic.
Clinicians should be aware that certain clinical features such as obesity, nail psoriasis, and intergluteal cleft site involvement in psoriatic patients might be associated with an increased risk of PsA development.
The joint Dermatology-Rheumatology clinic is the optimal solution for patients with psoriasis and PsA to improve care for this patients and minimalize disease consequences.



