Introduction
VEXAS syndrome is an adult-onset autoinflammatory disorder described for the first time in December 2020 [1]. The name VEXAS is an acronym from: Vacuoles (commonly identified in the bone marrow stem cells of the patients); the E1 ubiquitin conjugating enzyme is altered due to mutation of the UBA1 gene located in the X chromosome; the disease is an Autoimmune condition, and the disease is acquired and results from Somatic mutation. The clinical picture of VEXAS syndrome is broad and heterogeneous, and a number of symptoms and signs associated with the musculoskeletal system resemble those of other rheumatic diseases.
VEXAS syndrome was reported for the first time by David B. Beck from the National Human Genome Research Institute (National Institutes of Health, Bethesda, MA) and a co-working group of clinicians and scientists representing various centers. The syndrome was discovered through analysis of a large database, and the first was a genotype approach [1]. The group investigated the genome of 2,560 individuals, including almost 1,500 of those with undiagnosed recurrent fever and 1,083 patients with atypical disorders. They identified 25 males with a somatic mutation of the UBA1 gene. Three of them had a previously unreported somatic mutation at codon 41. The mutation was suggested on the basis of in silico studies to be harmful.
Further insight and investigations of other cohorts led to the discovery of a larger group of patients with clinical symptoms like those of the original three patients, and the syndrome was established as a new clinical entity [2]. Since its discovery more than 165 papers addressing the VEXAS syndrome have appeared in print, and several new cases have been reported.
VEXAS syndrome is an autoinflammatory systemic disease caused by somatic, i.e. acquired monogenic, mutation of the UBA1 gene coding the E1 enzyme initiating ubiquitination of several proteins. The clinical picture of VEXAS syndrome is heterogeneous and is not limited to hematologic phenomena (including myelodysplastic syndrome). The syndrome is characterized by systemic features (fever, fatigue), chondritis resembling symptoms of relapsing polychondritis, various dermatologic manifestations, including erythema nodosum, arthritis and myositis, pulmonary infiltrations, ocular involvement as well as various forms of vasculitis.
The review was intended to summarize reports of VEXAS syndrome with a focus on symptoms and signs akin to those of rheumatic diseases.
Pathogenesis
Somatic mutation of the UBA1 gene is considered as the etiopathogenic mechanism causing VEXAS syndrome. The UBA1 gene is located on chromosome X in position Xp11.23. Four types of mutation have been reported. The most common (about 45%) mutation results in substitution of methionine residues with threonine in position 41. Less common (about 30%) is the mutation which affects a single nucleotide (121 adenosine > guanosine) and it results in substitution of a methionine residue with a valine residue in position 41 of the enzyme polypeptide chain. In about 18% of patients with VEXAS syndrome substitution of methionine to leucine residues in the same position results from substitution of adenosine with cytosine [3].
Other mutations, including splice mutation within intron 2, have also been reported, and the problem of genetic alterations causing VEXAS syndrome is still a subject of investigations [4]. Recently, a new mutation was reported in the Czech Republic [5]. It should also be mentioned that hereditary mutations in UBA1 are associated with X-linked spinal muscular atrophy type 2.
The UBA1 gene codes one of the enzyme isoforms of ubiquitin-like modifier activating enzyme 1. This isoform is called nuclear in the contrast to isoform 2 detected in the cytoplasm and known as the cytoplasmic isoform. Ubiquitins are small regulatory proteins, with a molecular mass of 8.6 kDa. UBA1 is responsible for activation of ubiquitin 1. The proteins bind to other proteins in a process called ubiquitination. Ubiquitination is important for metabolic processing of the proteins. It can mark proteins for degradation via the proteasome, alter their cellular location, affect their activity, and modify protein interactions. Loss of ubiquitination has been suggested as a mechanism of so-called endothelial reticulum stress and can be associated with activation of the unfolded protein response. The suggested pathomechanism is a systemic phenomenon and it explains a variety of clinical symptoms and signs.
Somatic mutation leading to VEXAS syndrome was detected in myeloid medullary progenitor cells. This is a link between myelodysplastic syndrome and autoinflammatory conditions.
Several aspects of development of clinical characteristics of VEXAS syndrome remain unclear. It is not known how the cytopenias develop in the patients and relapsing inflammation progresses. It is hypothesized that a number of heterogeneous signs of the syndrome are secondary to inflammation and develop in patients with some predispositions to various forms of response to inflammation. Some studies indicated overproduction of such cytokines as interferon-γ, interleukin-8 and interferon-inducible protein 10. The mechanism of these phenomena and their role in development of clinical manifestations of the syndrome remain unknown.
Epidemiology
Predominance of VEXAS syndrome was estimated from analysis of available genetic data. Data collected from more than 163,000 participant of the Geisinger My-Code Community Health Initiative suggested that the syndrome occurs with prevalence of 1 : 14,000 in the whole investigated population, and is much more common in males (1 in 4,000 for males over 50 years) [6, 7]. The recent studies indicated that the VEXAS syndrome is more common than previous estimations suggested [8].
The syndrome is associated with a genetic mutation on chromosome X. It is clear that this autoinflammatory disease occurs predominantly in men. Clinically overt signs of the syndrome are seen in patients over 50 years. Very few female patients have been reported. The disease in female patients results from monosomy X, Turner syndrome or somatic mosaicism [6].
Clinical manifestations
General and systemic manifestations
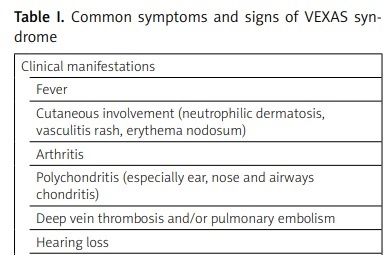
VEXAS syndrome is associated with several non-specific symptoms. The most common are intermittent fevers, fatigue and malaise as well as weight loss which cannot be explained by any detectable cause. The beginning of an overt disease in some patients is similar to early stages of autoimmune disorders. The autoantibodies are detectable in only a very few patients. The initial stage lasts various durations and subsequently other manifestations become detectable [2]. The most common clinical manifestations of the syndrome are summarized in Table I.
Table I
Common symptoms and signs of VEXAS syndrome
Polychondritis
Polychondritis or localized chondritis is one of the most common features of VEXAS syndrome. Auricular or nasal chondritis is an organ manifestation reported in about 60% of patients [9, 10]. In the majority of cases, the chondritis in the patients with VEXAS syndrome is indistinguishable from relapsing polychondritis. It was the reason to investigate the frequency of the UBA1 mutation in patients suffering from relapsing polychondritis. The results vary in a broad range (7% to 73%) [11–13]. The reason for the variability remains poorly understood but it is possible that different populations of patients with relapsing polychondritis were investigated, and also racial differences were suggested [14, 15].
Ferrada et al. [10, 16] reported that more than 50% of patients with VEXAS syndrome suffered from a clinical condition that fulfills criteria to diagnose relapsing polychondritis. They investigated the subset of patients with relapsing polychondritis and UBA1 mutation.
The patients of the VEXAS subset as compared to those with relapsing polychondritis without UBA1 mutation were all male with disease onset in the fifth decade of life or later, and had ear chondritis associated with fever, skin involvement, deep venous thrombosis and pulmonary infiltrations. Acute phase reactants were elevated and the patients had hematological abnormalities distinctive for VEXAS syndrome. It is interesting that the VEXAS patients had no chondritis of the airways or costochondritis. Prognosis of the VEXAS subset patients was worse, and their mortality was almost five times higher than in those with relapsing polychondritis without UBA1 mutation.
Ferrada et al. [16] proposed an algorithm for distinction of the VEXAS subset of patients based on male sex, a mean corpuscular volume >100 fl, and a platelet count < 200 × 103/μl.
Vasculitis
Vasculitides are a common manifestation of VEXAS syndrome. The most common is small-vessel vasculitis in the form of leukocytoclastic vasculitis [17–19]. Histopathologic evaluation of skin biopsy from the patients with the syndrome revealed this form of vasculitis in 80–90% of patients. Most of them had angiocentric segmental inflammatory infiltrations, commonly composed of neutrophils and fibrinoid necrosis. Forms of vasculitis other than leukocytoclastic were reported in VEXAS patients as well, including IgA vasculitis and ANCA-associated vasculitis (granulomatosis with polyangiitis or microscopic polyangiitis). It is unknown whether these conditions are related to the pathogenesis of the syndrome or are a coincidental disease.
Watanabe et al. [9] summarized nine reported cases of medium-size vasculitis in patients with VEXAS syndrome. Seven patients suffered from polyangiitis nodosa. Large vessel vasculitis in patients with the syndrome seems also to be rare, and was described mostly in form of giant cell arteritis [20].
Thrombosis is a common manifestation of VEXAS syndrome. Some reports indicated that this complication occurs in about 40–56% of patients [21, 22]. The pathomechanism of thrombosis is probably complex and varies from cases to case. A high level of factor VIII or factor IX in the serum of VEXAS patients was reported [23] and lupus anticoagulant or antiphospholipid antibodies were described [2].
Other musculoskeletal involvement
Arthritis in two relatively large case-series was reported to be a frequent manifestation. Ferrada et al. [16] found joint inflammation in 58% and Georgin-Lavialle et al. [12] reported arthritis in 28.4%. The details and location of the arthritis were not described. In most case descriptions, arthralgias and arthritis occurred in the large and medium joints and were not specific for any distribution pattern. Some authors reported “fever-associated arthritis”, and this description referred to a low-degree inflammation accompanying fever [24]. Fever and arthralgia were also reported in a patient with the syndrome mimicking systemic lupus erythematosus [25].
Myositis may be severe and pathological findings indicate myofascitis with macrophagic infiltrations and necrotizing myopathy [2]. In some cases, intramuscular vacuoles were observed [26]. The myositis was prominent in the form of orbital myositis and this clinical picture (with chondritis, systemic inflammation and arthritis) was suggested to be a new phenotype of the syndrome.
Cutaneous manifestations
Skin involvement is very common and is reported in more than four-fifths of all patients with VEXAS syndrome. Cutaneous manifestations are very heterogeneous. The most common signs of vasculitis, neutrophilic dermatitis, erythema nodosum and erythematosus papules are reported in the patients. Less frequently cutaneous involvement includes urticaria, Sweet syndrome-like nodules, periorbital edema, and injection-site reactions. Rarely purpuric macules, eczematous rash and firm, tender infiltrates are detected in the patients [26].
Heterogeneity of the skin involvement suggests various mechanisms responsible for its development. Pathological evaluation of a skin biopsy revealed leukocytoclastic vasculitis affecting small (and in some cases, medium) vessels. Cellular infiltrates were observed and consisted of various kinds of cells (mixed cell type) but infiltrates containing a single-cell composition were also found. These infiltrates were built up with lymphocytes, eosinophils or neutrophils [12, 27].
Hematologic abnormalities
The hematologic component of the clinical picture of VEXAS syndrome is important both from diagnostic and therapeutic points of view. Almost all patients presented macrocytic anemia [28]. Other cytopenias are also frequent; lymphopenia was reported in 80% of the patients, and monocytopenia and thrombocytopenia occur in a half of all VEXAS patients [3].
A significant clinical problem is a common association of the syndrome with hematological abnormalities leading to malignant conditions [29]. About a half of the patients have myelodysplastic syndrome. It is consistent with earlier suggestions of an association of myelodysplastic syndrome with autoimmune disorders [30]. More detailed studies revealed that myelodysplastic syndrome in patients with VEXAS is in some aspects different from the “classical” form of myelodysplasia. Myelodysplastic syndrome occurs predominantly in a similar population to VEXAS, i.e. men older than 70 years, and results from clonal alterations of hemopoietic stem cells responsible for inefficient hematopoiesis. In about 30% of patients the syndrome is transformed to acute myeloid leukemia. It is believed that clonal proliferation of hematopoietic cells modifies the marrow microenvironment, resulting in cytopenias. A number of acquired genetic alterations were linked to myelodysplastic syndrome.
In patients with VEXAS syndrome the profile of mutations causing myelodysplasia is limited and still insufficiently investigated. It remains unclear how the UBA1 mutation may affect the development of myelodysplastic syndrome. Management of myelodysplasia in VEXAS patients is also similar to general hematological management [3, 9].
Ocular manifestations
Ocular involvements are common and are estimated to be present in about 40% of patients. They have various nature, akin to ocular involvement in patients with systemic diseases of connective tissue, especially vasculitides. Abnormalities within the eyes include uveitis, scleritis and episcleritis as well as periorbital inflammation, most commonly in the form of blepharitis [2].
Other organ manifestations
Cardiac manifestations are found in about one-tenth of patients in the form of myocarditis and pericarditis [7]. Myocarditis can evolve into a cardiomyopathy. Vasculitis of the cardiac arteries can provoke ischemic heart disease. A case of acute high-output cardiac failure caused by an arteriovenous fistula with a common iliac artery aneurysm was recently reported [31].
Pulmonary manifestations are relatively common [32]. Almost half of the patients have pulmonary infiltrates. Less common are pleural effusion and idiopathic interstitial pneumonia. Single case reports of bronchiolitis obliterans, pulmonary vasculitis, bronchiectasis, and alveolar hemorrhage have been published [7, 32].
Diagnosis and differential diagnosis
The altered myeloid progenitor cells, predominantly granular and erythroid precursor cells were shown to contain vacuoles. The vacuoles are not pathognomonic for VEXAS syndrome and are a nonspecific sign of cellular damage only [33, 34]. Templé and Kosmider [6] suggested that occurrence of the vacuoles in more than 10% of cells in a patient with a clinical picture akin to VEXAS syndrome is an indication for further genetic studies on UBA1 gene mutations.
Treatment
There is no standardized recommended therapeutic strategy in VEXAS patients. Most of the details are obtained from observation of either short case series or single case reports. In general, the medication is focused on inhibition of systemic inflammation and eradication of the UBA1-mutated population of hematopoietic cells. Additionally, symptomatic treatment of organ manifestations should be applied. Thus, the management of VEXAS patients is a multidisciplinary approach and requires a team of specialists including a rheumatologist and a hematologist.
Most of the observations and retrospective analyses indicate transient effectiveness of high doses of glucocorticosteroids [2]. After obtaining some control of inflammatory symptoms dosage tapering is usually associated with flares or glucocorticosteroid-side effects resulting in a need to decrease the dose of the medication. Initially useful in the disease control, glucocorticosteroid are to be substituted by glucocorticosteroid-sparing treatment.
The interleukin-1 receptor antagonists were found to be effective only in some patients and commonly induced skin reactions at the site of injection. Combination of anakinra and cyclosporine A was proposed as therapy avoiding the undesirable cutaneous reaction [35]. Unfortunately, the drug combination may cause neutropenia. Tocilizumab, an interleukin-6 inhibitor, was shown to be effective in some patients only [7].
Some authors indicated the use of Janus kinase inhibitors as a medication with several pathways of activation of the systemic inflammation.
Heiblig et al. [36] performed a retrospective multicenter study on 30 VEXAS patients and revealed that ruxolitinib is more effective than other drugs of this class. Ruxolitinib is a Janus kinase inhibitor with selectivity for subtypes JAK1 and JAK2. It is approved for the treatment of intermediate or high-risk myelofibrosis, polycythemia vera, and glucocorticosteroid-refractory acute graft-versus-host disease. Administration of the other Janus kinase inhibitors leads to discrepant results [6].
It is important to highlight the variability of the reaction of VEXAS patients to anti-inflammatory medication. It is possible that there are subtypes of the syndrome and in future the therapeutic strategy should be tailored according to the disease subtype or type of mutation.
Hematological management of VEXAS patients is based on administration of azacytidine, a hypomethylating agent [37]. This medication was suggested to be an effective measure especially in patients with concomitant myelodysplastic syndrome. The drug is a chemical analogue of cytidine, a nucleoside which builds up the nucleic acids. Azacytidine at low doses inhibits DNA methyltransferase and is a hypomethylating agent, and at high doses is directly cytotoxic to abnormal hematopoietic cells [4].
Autologous hematopoietic stem cell transplantation is considered as a curative intervention when the syndrome is diagnosed at an early stage. Reports of this therapy are still limited [2, 4]. The role of cell transplantation as a possible treatment for VEXAS patients is a subject of a long-term project initiated in 2022 [38].
Prognosis
Mortality in VEXAS patients is high. The five-year survival rate is 63%. Other reports indicated 50% mortality within 3.96 years. The main cause of death is disease progression [2].
Disease registry
Currently, the increased interest and knowledge about VEXAS has also stimulated the creation of a network of clinical and scientific centers dealing with this topic and the creation of registries of patients diagnosed with this syndrome. An example is the AIDA Registry, which was created thanks to the AIDA Network and enabled the cooperation of 113 centers from 23 countries [39]. An international registry seems necessary for a better understanding of the pathogenesis and development of optimal recommendations for the diagnosis and treatment of this rare and still difficult to diagnose disease.
Conclusions
VEXAS syndrome is not only a new clinical entity. It is a prototypic disease of hematoinflammatory disorders that have been proposed as a new class of rheumatic diseases. Hematoinflammatory diseases are caused by somatic mutation in the blood cells associated with multiorgan involvement and bone marrow pathology. Bone marrow abnormalities are associated with proliferation (lymphoproliferation, myeloproliferation) or myelodysplasia and potentially can transform into malignancy.
The concept of hematoinflammatory diseases confirms the role of somatic mutations limited to a group of non-germline cells and indicated a link between inflammatory, i.e. autoinflammatory, diseases and bone marrow abnormalities. It has been suggested that a rare form of histiocytosis, Erdheim-Chester disease, resulting from mutations of a proto-oncogene, is also a disorder from the new class.
From a clinical practice point of view, the discovery of VEXAS syndrome sheds light on a relatively common problem of patients with fever of unknown origin. To a long list of causative mechanisms of fever, VEXAS syndrome added another, with several signs and symptoms mimicking previously known rheumatic diseases. Such patients are met in consultations of unclear cases referred to rheumatologists, and understanding of the problem and access to diagnostic methods (including genetic investigations) are crucial. There is no doubt that this problem is a field of further investigations carried out by international teams including Polish researchers.