Introduction
Systemic vasculitis is a commonly unrecognized cause of coronary artery stenosis and myocardial ischemia [1]. One of the primary vasculitides in which the coronary arteries may be affected and which cause coronary artery disease (CAD) in young patients is Takayasu arteritis (TAK) [2, 3].
Takayasu arteritis is a granulomatous vasculitis affecting large-diameter vessels, predominantly the aorta and its proximal branches. This condition is more commonly found in young women [4]. In the past, scientists used different terminology to describe this condition – “pulseless disease” or “aortic arch syndrome”.
The involvement can be segmental or extend to the entire thoracic and abdominal aorta and its branches [5]. In TAK, as mentioned above, coronary arteries may also be involved in the pathological process [5, 6]. Takayasu arteritis is associated with significant morbidity and mortality in young patients [7], and cardiac involvement is one of the leading causes of morbidity and mortality in TAK patients [8, 9].
Coronary artery lesions (CAL) worsen the prognosis of TAK patients [6, 10, 11] due to the nonspecific clinical manifestations, delayed diagnosis, and the difficulty of treatment [10, 11]. Khor et al. [12] note that the average duration of the disease before the diagnosis of TAK was 66 months (interquartile range 33–177 months).
Coronary artery lesions is one of the leading causes of morbidity and mortality in TAK patients [8, 13, 14], emphasizing the importance of early diagnosis and treatment of this pathology [14]. There are still no known specific risk factors for CAL, making it crucial to identify risk factor and clinical characteristics of TAK with CAL and TAK involving coronary arteries. Timely identification of high-risk groups and implementation of effective early interventions are essential for improving patient outcomes [15].
Data on the epidemiology of TAK are limited [4]. The annual incidence of TAK is reported to be 1–2 cases per 1 million population [16]. This condition occurs predominantly in individuals under 50 [5, 17], mainly affecting women from the Mediterranean area, Southeast Asia, and the Middle East [5]. According to Sun et al. [14], the female-to-male ratio is 4 : 1, and another study conducted in Malaysia reported a female-to-male ratio of 12 : 1 [12]. In a study conducted in China, the average age of TAK patients was 36.03 ±12.70 years [18].
The etiology of TAK remains insufficiently understood [19, 20]. In the development of TAK, factors such as infection, including Mycobacterium tuberculosis and Chlamydia pneumoniae, and genetic factors (association with the HLA complex) may play a role [15, 20].
The pathogenesis of TAK still needs to be fully clarified [15]. Various immune mechanisms, both cell-mediated and humoral, leading to inflammation and tissue damage, are involved in the pathogenesis of TAK [15, 20, 21]. Hyperproduction of cytokines [22] and levels of sex hormones [15, 23] also play a role.
In TAK, inflammation and intimal proliferation lead to thickening of the arterial wall, stenosis or occlusion of the artery lumen, thrombosis, and the destruction of the elastic and muscular layers, contributing to the formation of aneurysms or the development of dissection [20].
The review aimed to analyze contemporary literature data regarding the prevalence, development mechanisms, risk factors, clinical features, diagnosis, treatment, and prognosis of CAL in patients with TAK.
The goal is to enhance the awareness of family physicians, cardiologists, and rheumatologists regarding the manifestations of TAK because early diagnosis, appropriate immunosuppressive treatment, and, when necessary, revascularization can improve the prognosis of patients.
Material and methods
The literature analysis was independently performed by two authors for the period from 2003 to 2023 using Scopus and MEDLINE/PubMed searches. Search queries included various terms and their combinations: “Takayasu arteritis”, “coronary angiography”, “myocardial revascularization”, “coronary artery disease”, “angina pectoris”, and “myocardial infarction”.
The analysis included literature reviews, case-based reviews, and clinical case descriptions. Sixty-nine articles published in English were selected for the final analyses.
Clinical manifestations of Takayasu arteritis
The clinical manifestations of TAK are diverse and depend on the stage of the disease [20] and the localization of the affected arteries [19, 23]. In the early, active inflammatory stage, nonspecific systemic symptoms are characteristic, often overlooked, or considered signs of more common acute illnesses.
The late, chronic stage (the “pulseless” stage) is characterized by ischemia and symptoms secondary to arterial occlusion [20]. The clinical symptoms of TAK can range from mild to severe, life-threatening complications [23].
Clinical manifestations of TAK include constitutional symptoms (fatigue, malaise, headache, dizziness, fever, arthralgia), carotidynia, intermittent claudication, weakened/absent pulses in radial arteries, vascular murmurs, different blood pressure in limbs, and arterial hypertension (AH) [12, 18, 24]. As noted by Huo et al. [23], acute myocardial infarction (AMI) and stroke are the most serious complications of TAK, leading to the development of severe heart failure (HF), malignant arrhythmias, neurological defects, and even life-threatening situations.
Cardiovascular lesions
Cardiovascular pathology is observed in 39.9–57% of patients with TAK [10, 25]. Any anatomical structure of the heart can be involved in the pathological process: pericardium, heart valves, myocardium, and coronary arteries [10, 14].
Cardiovascular involvement may manifest as AH, aortic valve insufficiency, mitral and tricuspid insufficiency (rarely), ischemic heart disease (IHD), myocarditis, pericarditis, dilated cardiomyopathy, HF, and the formation of intracardiac thrombi [6, 24, 26–28].
It is worth noting that in TAK, the pulmonary artery can also be involved in the pathological process, leading to the development of pulmonary hypertension. Mukoyama et al. [27] found that patients with pulmonary artery involvement more often had IHD than those without it (29% vs. 9.3%; p = 0.018).
According to the study by Goel et al. [7], patients with TAK had an increased risk of developing IHD, stroke/transient ischemic attack, combined cardiovascular diseases (CVD), and peripheral vascular diseases compared to the control group. However, there was no observed increase in the risk of AH, chronic kidney disease, HF, or diabetes. Park et al. [9] emphasized that timely diagnosis of cardiac involvement is crucial, as this pathology was the most common cause of morbidity and mortality in these patients.
Coronary artery lesions
Mechanisms of CAD in TAK include in situ coronary artery inflammation or extension from adjacent aortitis [26]. Additionally, in patients with TAK, coronary artery pathology may develop not only due to inflammation [26, 29] but also as a result of accelerated atherosclerosis [26], endothelial dysfunction [30], and other factors.
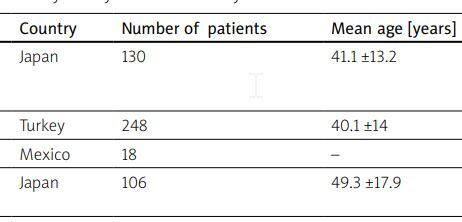
Coronary artery lesions are a distinct but not uncommon type of artery involvement in TAK, observed in 5.9–58.2% of patients according to visualization methods (Table I) [3, 31–32].
Table I
Frequency of coronary artery lesions in Takayasu arteritis
| Research | Country | Number of patients | Mean age [years] | Frequency of CAL (%) |
|---|---|---|---|---|
| Endo et al. 2003 [35] | Japan | 130 | 41.1 ±13.2 | 38.3 (81 patients underwent selective coronary angiography) |
| Bicakcigil et al. 2009 [25] | Turkey | 248 | 40.1 ±14 | 8.9 |
| Soto et al. 2011 [36] | Mexico | 18 | – | 44.4 |
| Ohigashi et al. 2012 [37] | Japan | 106 | 49.3 ±17.9 | 5.9 (< 39 years); 28.6 (> 40 years) |
| Sun et al. 2013 [14] | China | 587 | 43.5 ±13.5 (of 45 patients with CAL) | 7.7 |
| Kang et al. 2014 [33] | South Korea | 111 | 44.0 ±13.8 | 53.2 |
| Yang et al. 2014 [11] | China | 566 | 36.3 (range 5–86) | 11.7 |
| Comarmond et al. 2014 [38] | France | 27 (and 80 in control group) | 48.0 (range 34–61) | 25.9 |
| Yuan and Lin 2020 [3] | China | 141 | 35.7 ±16.9 37 (range 0.58–73) years (n = 67) | 58.2 |
| Danda et al. 2021 [39] | India | 602 | 15 (range 12–18) 30 (range 25–38) | 16 |
| Xu et al. 2023 [31] | China | 66 | 85.2 (age of onset age range 12.0–133.8), months | 39.4 (in children) |
| Ci et al. 2023 [32] | China | 115 | 41.40 ±12.27 | 35.7 |
Results from coronary computed tomography angio-graphy (CCTA) showed CAL in 53.2% of TAK patients, but cardiovascular symptoms were observed in only 33.9% of patients [33]. Yuan et al. [3] observed CAL in 58.2% of patients, and in 41.8% of patients involvement of other aortic branches was found.
However, the authors acknowledge that these results may be influenced by patient selection bias. Conversely, other researchers point out that since coronary angiography was not routinely performed in TAK patients, the frequency of CAL may be underestimated [10].
Since coronary artery stenosis and occlusion are more common in the mouths and proximal segments of the coronary arteries [11, 14, 23, 34–36], and subsequent myocardial ischemia is one of the causes of death in patients with TAK, early detection of CAL, especially before occlusion develops, is important to reduce mortality [10].
Versini et al. [29] found that the proportion of IHD in patients with TAK was higher than in the control group (32.3% vs. 8.9%; p < 0.001). In multivariate analysis, IHD was associated with TAK (odds ratio, OR = 6.576, 95% confidence interval, CI: 4.09–10.64) and male sex (OR = 2.29, 95% CI: 1.43–4.26). In another study, the adjusted risk ratio of IHD in patients with TAK compared to the control group was (adjusted hazard ratio, HR = 3.08, 95% CI: 1.47–6.44; p = 0.003) [7].
Ohigashi et al. [37] reported that the incidence of IHD in adult patients with TAK increases with age. According to their findings, patients with TAK with CAL were significantly older than those without CAL (52.54 ±11.17 vs. 37.73 ±12.72; p < 0.001). They had a higher age of onset of the disease (42.21 ±11.46 vs. 32.74 ±13.13; p < 0.001) and a longer course of the disease (p < 0.001).
Additionally, they had a higher body mass index (BMI, p = 0.002) and higher rates of smoking, alcohol consumption, diabetes mellitus, and dyslipidemia (p < 0.05), as well as higher levels of uric acid and triglycerides (p < 0.05). Other authors noted that risk factors for TAK with CAL included the age of TAK onset (OR = 1.143, 95% CI: 1.007–1.298; p = 0.039), the course of TAK (OR = 1.165, 95% CI: 1.025–1.324; p = 0.020), and BMI (OR = 1.100, 95% CI: 1.021–1.185; p = 0.013) [15].
The study also revealed a significant difference in the average duration of the disease between patients with CAL (176 months; range 13–282 months) and those without CAL (21 months; range 1–142 months) (p = 0.013) [36].
Involvement of the coronary arteries in TAK leads to the development of stable angina pectoris (AP) corresponds to the term: stable coronary artery disease (SCAD), unstable angina pectoris (unstable coronary artery disease – UCAD), myocardial infarction (MI), and sudden death [1, 2, 19, 24, 38–42]. Table II shows the frequency of AP in patients with TAK and CAL.
Table II
Frequency of angina pectoris in patients with Takayasu arteritis
| Research | Country | Number of patients | Mean age [years ±SD] | Female (%) | Frequency of angina pectoris (%) |
|---|---|---|---|---|---|
| Bicakcigil et al. 2009 [25] | Turkey | 248 | 40.1 ±14 | 89.1 | 17 |
| Sun et al. 2013 [14] | China | 45 | 43.5 ±13.5 | 80.0 | 88.9 |
| Yoshida et al. 2016 [42] | Japan | 86 | 36.4 ±18.6 (age at onset) | 91.9 | 10.5 |
| Lei et al. 2020 [24] | China | 38 | 32.9 ±2.4 | 78.9 | 13.2 (stable angina); 44.7 (unstable angina) |
| 9 (TAK pediatric) | 14.3 ±3.3 | 88.9 | 0.0 (stable angina); 22.2 (unstable angina) | ||
| 29 (TAK adult) | 38.6 ±12.0 | 75.9 | 17.2 (stable angina); 51.7 (unstable angina) | ||
| Yuan and Lin 2020 [3] | Japan | 141 | 35.7 ±16.9 | 77.3 | 36.3 |
| Huo et al. 2022 [23] | China | 94 | 36.5 ±12.3 (the mean age at symptom onset), 38.8 ±12.3 (mean age of onset of cardiac ischemia) | 85.1 | 72.3 |
Soto et al. [36] observed typical AP in 62.5% of patients with CAL, atypical AP in 12.5%, and dyspnea in 25%. Shimizu et al. [43] described TAK with isolated CAL in a 55-year-old woman with heterozygous familial hypercholesterolemia who complained of exertional AP.
As coronary angiography revealed ostial left anterior descending coronary artery (LAD) stenosis, the patient underwent directional coronary atherectomy followed by a drug-coated balloon. However, after 6 months, restenosis occurred at the ostial LAD, and the ostial left circumflex coronary artery (LCX) progressed significantly.
Intravascular ultrasound (IVUS) findings showed that the media was partly unrecognizable, and an echo intensity similar to fibrotic intimal thickening traversed from the intima to the adventitia, causing the whole image of the coronary artery to be unclear.
The patient underwent repeated directional coronary atherectomy followed by drug-coated balloon procedures for both LAD and LCX; however, after five months, the involvement of the LAD and LCX progressed, leading to coronary artery bypass grafting (CABG). Based on histological examination of coronary artery samples (excessive fibrous thickening of the intima and adventitia, with granulomatous inflammation in the media), a diagnosis of isolated coronary TAK was established.
Another case was described involving the development of progressive AP in a 24-year-old woman as the first manifestation of TAK. Coronary angiography revealed severe stenosis of the left coronary artery, and immediate percutaneous coronary intervention (PCI) with implantation of an everolimus-eluting stent was performed due to sAP and signs of acute myocardial ischemia on the electrocardiogram (ECG).
Takayasu arteritis was suspected based on the detection of hypoechogenicity on post-procedural IVUS. Despite treatment with high doses of prednisolone and tocilizumab (TCZ; anti-IL-6 receptor antagonist), the patient experienced recurrent AP after 5 months.
Repeat coronary angiography revealed stent restenosis, and despite the administration of high-dose prednisolone and TCZ, stent restenosis persisted, so CABG was performed [1].
It has been established that CAL in children and adults has differences, with coronary artery stenosis being the most common in adults, while children exhibited dilation of the coronary arteries. Xu et al. [31] observed CAL in 39.4% of children with TAK diagnosed using ultrasound, and none of them had AP or signs of ischemia on the ECG.
In 73.1% of children, CAL developed within 36 months of disease onset, and none of the children exhibited MI. The left main coronary artery (LMCA) (84.6% of cases), right coronary artery (RCA) (73.1%), and LCX (65.4%) were involved in the pathological process, showing dilation without stenosis, occlusion, or fistula. Most lesions were small or middle coronary artery aneurysms, with five giant coronary aneurysmal dilations observed.
Additionally, beaded changes and mural thrombi were observed. Involvement of 1, 2, 3, and 4 arteries constituted 30.8%, 34.6%, 3.8%, and 30.7%, respectively. Notably, most CAL regressed or normalized under the influence of glucocorticosteroid (GC) treatment, other immunosuppressive agents, or biological agents.
Furthermore, after treatment, no difference in survival was observed between groups with and without CAL. Therefore, early detection of coronary artery pathology and appropriate treatment improved the prognosis in children with TAK. Researchers recommend evaluating coronary arteries at an early stage when diagnosing TAK in children, which is crucial for improving prognosis.
A case of TAK in an 11-year-old boy is described in the literature, where subsequent CCTA revealed severe stenosis (at least 95%) of the ostium of the RCA, and the left coronary artery was approximately 50% stenosed in the absence of collaterals. Notably, there were no symptoms of myocardial ischemia or changes on the ECG, highlighting the critical importance of imaging methods for coronary artery visualization in TAK [44].
It is worth noting that TAK is characterized by early and accelerated atherosclerosis development [26]. In a prospective study conducted in France, it was found that 87% of TAK patients had subclinical atherosclerosis, compared to 76% of patients with rheumatoid arthritis (p = 0.088) and 48% in the control group (p < 0.001) [45].
The results of Wang et al. [46] demonstrated that patients with active TAK had proatherogenic lipid profiles, and the ratio of apolipoprotein B (apoB) to apolipoprotein A1 (apoA1) could be used as a marker for monitoring and targeting TAK patients.
Additionally, patients with TAK had significantly increased carotid artery intima-media thickness compared to the control group (p = 0.0001) and significantly decreased flow-mediated dilation (p = 0.0001), suggesting pronounced endothelial dysfunction. The authors note that both chronic systemic inflammation and vasculitis can lead to endothelial dysfunction, atherosclerosis development, and IHD [30].
According to the results of Alibaz-Oner et al. [47], cardiovascular risk factors were more prevalent in TAK patients. Before the incidence/index date, the frequency of cardiovascular events (CVE) was significantly higher, and the Framingham 10-year general CV risk score was significantly higher in TAK patients compared to the control group.
After 10 years of observation, the cumulative incidence of CVE was 15.4% in the TAK group compared to 5.8% in the control group, and the risk of CVE was elevated among TAK patients (HR = 4.36, 95% CI: 1.25–15.13). These results indicate that patients with TAK should undergo a thorough assessment of CVD risk, and an aggressive approach to modifying CV risk is justified.
Comarmond et al. [48] investigated myocardial perfusion using thallium-201 myocardial scintigraphy in TAK patients and noted a high prevalence (84%) of myocardial perfusion defects, which decreased after dipyridamole administration. That suggests the reversibility of perfusion defects after vasodilator use, indicating a probable role of microcirculatory dysfunction in developing myocardial ischemia in TAK.
Myocardial infarction
Takayasu arteritis can cause coronary artery involvement in young women and adolescents [2, 41, 49]. Therefore, suspicion of TAK should arise in cases of chest pain in young women and adolescents [2, 49], facilitating early diagnosis of this condition and timely treatment of vasculitis, which reduces associated morbidity and mortality [2]. The frequency of MI in TAK ranges from 3.4% to 24.4% of cases [14, 50]. Additionally, Sun et al. [14] observed a history of MI in 8.9% of 15 patients. It is worth noting that MI can be the initial manifestation of TAK [41, 51].
In the literature, cases of MI complicated by cardiogenic shock resulting from 99% stenosis of the left main coronary artery (LMCA) have been described [52]. Wilson et al. [2] reported three cases of TAK with CAL in adolescent girls complicated by MI and angina, treated with balloon angioplasty, CABG, and heart transplantation. There is also a report of the sudden death of a 15-year-old girl, in whom autopsy revealed involvement of three major coronary arteries with multiple proximal lesions microscopically characteristic of TAK [19].
Diagnosis
Timely diagnosis of TAK is crucial, as delayed diagnosis can lead to significant morbidity [53]. Diagnosing TAK remains challenging due to its rarity, heterogeneous manifestations depending on the affected part of the artery, and nonspecific symptoms [2, 34].
Therefore, early diagnosis is essential for initiating effective treatment at the early stages of TAK and, if necessary, performing revascularization procedures to improve patient outcomes. Characteristic symptoms of TAK include disease onset before age 40, intermittent claudication, vascular bruits, asymmetric pulses, and high blood pressure [16].
As noted in the European Alliance of Associations for Rheumatology (EULAR) recommendations (2018), most TAK symptoms are nonspecific, but their presence should prompt a thorough examination of the arteries. Since there is no gold standard for TAK diagnosis, it is recommended to refer the patient to an experienced center for further evaluation, including visualization of large-diameter vessels [54].
It is essential to emphasize that the diagnosis of TAK is based on the updated (2022) American College of Rheumatology/EULAR classification criteria [55].
Discussion
Patients with TAK often exhibit elevated levels of inflammatory markers, including C-reactive protein and erythrocyte sedimentation rate. However, the systemic inflammatory response does not always correlate positively with the inflammatory activity in the vessel wall. Hence, imaging studies play a crucial role in diagnosing and monitoring the disease [56]. Studies have shown that children with TAK with CAL have higher levels of leukocytes, platelets, C-reactive protein, tumor necrosis factor-α (TNF-α), and interleukin-2 receptor (IL-2R) compared to those without lesions [31].
Chen et al. [18] investigated homocysteine (HCY) and lipid levels in serum and analyzed CAL in patients with TAK with active disease. Patients with TAK had significantly higher HCY levels than healthy individuals (p < 0.0001), and those with active TAK had higher levels than those with inactive TAK.
Levels of low-density lipoprotein cholesterol (LDL-C), the ratios of LDL-C to high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC) to HDL-C, and triglycerides (TG) to HDL-C, as well as the values of the atherogenic index of plasma (AIP), were significantly higher in patients with active TAK compared to those with inactive TAK, and in patients with TAL with CAL compared to those without CAL.
Conversely, serum HDL-C levels were significantly lower in patients with active TAK compared to those with inactive TAK and in patients with CAL compared to those without lesions (p < 0.05). Additionally, TC and TG serum levels were significantly higher in patients with TAK with CAL than those without CAL. The authors found that independent risk factors for CAL were HCY levels, TG, and the TG/HDL-C ratio.
Elevated serum HCY levels increased the risk of CAL by 1.3 times (OR = 1.275, 95% CI: 1.056–1.539; p = 0.011), serum triglycerides increased the risk by 3.5 times (OR = 3.534, 95% CI: 1.907–6.547; p < 0.0001), and the risk of CAL was 2.5 times higher with an elevated TG/HDL-C ratio (OR = 2.513, 95% CI: 1.567–4.032; p < 0.0001).
Instrumental diagnosis
According to the EULAR recommendations (2018), magnetic resonance imaging (MRI) is the first-line investigation method for TAK, which is the optimal method for assessing inflammation of the vessel wall and changes in the vessel lumen, and, importantly, it does not involve radiation.
Alternative examination methods include computed tomography (CT), positron emission tomography (PET), and ultrasound (US). Conventional angiography is not recommended for diagnosing TAK, as the visualization methods mentioned above have replaced it. Imaging methods may confirm or exclude activity, and their use is not recommended in remission. Magnetic resonance imaging angiography (MRA), CT angiography, and US can be used for long-term monitoring of structural damage, including diagnosing stenosis, occlusion, dilation, and/or aneurysms.
Based on the results of coronary CCTA and coronary angiography, the most common type of vessel involvement was Numano type V (61.6%), followed by type IIb (18.95%), IV (8.95%), I (4.7%), III (3.7%), IIa (2.1%), and in patients with coronary artery stenosis ≥ 50%, type V (18%). In patients with TAK and cardiac involvement, type V vessel involvement, according to the Numano classification, was observed most frequently (67.1%) [10, 57].
Cox regression (or proportional hazards regression) analysis showed that vessel involvement of type V was significantly associated with an increased risk of CVE (documented CAD: MI, AP, revascularization) (adjusted HR 2.852, 95% CI: 1.474–5,518; p = 0.002), while the use of methotrexate (MTX) was associated with a reduced risk of CVE (adjusted HR 0.515, 95% CI: 0.268–0.993; p = 0.047) [58].
Results from a retrospective study in China showed that coronary artery pathology with type V Numano vessel involvement was more common, accounting for 33.8% of cases, compared to non-type V vessel involvement at 17.4% (p = 0.025). Additionally, involvement of the LAD and LCX was more frequently observed in patients with type V Numano TAK compared to those without type V involvement (LAD 76.0 vs. 41.7%, p = 0.041; LCX 56.0 vs. 8.3%; p = 0.006, respectively) [59].
Literary data (Table III) from imaging methods of coronary arteries indicate that the most common localization of CAL is at the ostia and proximal segments of coronary arteries [14, 36], leading to stenosis or occlusion [3, 18].
Table III
Characteristics of coronary artery involvement in Takayasu arteritis based on visualization studies
| Research | Country | Number of patients | Mean age, years | Involvement of coronary artery ostia (%) | Involvement of proximal segments of coronary arteries (%) | Involvement of middle segments of coronary arteries (%) | Involvement of distal segments of coronary arteries (%) | Stenosis/occlusion (%) | Dilation/aneurysm (%) |
|---|---|---|---|---|---|---|---|---|---|
| Endo et al. 2003 [35] | Japan | 130 (81 underwent selective coronary angiography; 31 patients had abnormal coronary angiographic findings) | 41.1 +13.2 | 87.5 (among 24 patients with coronary stenosis) | 12.5 (among 24 patients with coronary stenosis) | – | – | 77.4 | 9.7 (aneurysmal coronary ectasias), 9.7 (coronary artery – bronchial artery anastomoses), 3.2 (combined coronary ectasia and anastomosis) |
| Soto et al. 2011 [36] | Mexico | 18 | 36.8 +12.6 | 68.4 | 26.3 | 5.3 | – | – | – |
| Sun et al. 2013 [14] | China | 45 | 43.5 ±13.5 | 37.4 | 33.3 | 17.2 | 12.1 | – | 0 |
| Kang et al. 2014 [33] | Korea | 111 | 44.0 +13.8 | 28.0 | – | – | – | 36.9 (nonostial coronary arterial stenosis) | 8.1 |
| Yang et al. 2014 [11] | China | 566 | 36.3 ±13.4 | 35.7 | 26.7 | 23.3 | 14.3 | 80.3 | 7.6 |
| Lei et al. 2020 [24] | China | 38 | 32.9 ±2.4 | 44.7 | 55.3 | 57.9 | 34.2 | 97.4 | 15.8 |
| Huo et al. 2022 [23] | China | 94 (188 CAL) | 36.5 ±12.3 | 29.8 | 45.7 | 16 | 8.5 | 94.1 | 5.9 |
Broncano et al. [26] also demonstrated that coronary artery ostia and proximal segments were most frequently affected. Other forms of involvement, such as diffuse or focal stenosis of distal segments, aneurysms, or a combination known as the string of pearls sign, were less frequently observed.
Additionally, fistulas to other coronary or bronchial arteries were reported. Moreover, during active vasculitis, mural thickening with wall enhancement and a hypovascular halo were described on delayed CT angiographic images. Mural calcifications with mild thickening and enhancement may be present in inactive disease.
Koster et al. [16] note that specific signs on coronary angiography include stenosis of the ostia or proximal segments of coronary arteries and skip lesions. Another study found 188 coronary artery stenoses in 94 patients, with the most common site being the ostia and proximal segments of coronary arteries (75.5%) [23]. Endo et al. [35] reported that the coronary steal phenomenon was always associated with occluded pulmonary arteries and pulmonary hypertension.
Coronary CT angiography provides comprehensive information about coronary artery pathology in TAK. According to CCTA data, researchers observed coronary artery ostial or luminal stenosis of ≥ 50% or coronary aneurysms in 23.4% of patients with TAK and CAL [33].
According to the study, the LMCA was most commonly affected, found in 51.1% of patients, with stenosis of the LMCA lumen over 80% observed in 39.1% of cases.
In 48.9% of patients, involvement of one coronary artery was diagnosed; in 24.4% of patients, involvement of two coronary arteries; and in 26.7% of patients, involvement of three coronary arteries [14]. However, the results of a systematic review demonstrated that the RCA was more frequently involved (31.5% of cases), followed by the LMCA (28.6%) and LAD (23.2%) [3].
According to coronary angiography data, 207 coronary artery stenoses were identified in 87 patients with TAK. In men, more severe stenosis or occlusion of coronary arteries was observed compared to women (70.5% vs. 46%; p = 0.004), along with a higher risk of long-term mortality [6].
Intravascular US helps determine changes in the structure of the coronary artery wall and the presence of atherosclerotic plaques in coronary arteries [51]. Ishiyama et al. [52] reported that IVUS showed that the three-layered structure of the intima, tunica media, and adventitia was not visible, and the vessel was concentrically thickened; unstable plaque and calcification were not seen. Notably, the media was partly unrecognizable, and an echo intensity similar to fibrotic intimal thickening traversed from the intima to the adventitia, making the whole image of the coronary artery unclear [43].
Cardiac MRI using late gadolinium enhancement (LGE) revealed IHD in 25.9% of TAK patients, and a typical pattern of MI was observed in 22.2% of patients.
Although patients with TAK and CAD had a similar risk of CVE, the prevalence of myocardial ischemia was more than five times higher in TAK patients (p = 0.002). The authors note that the high prevalence of occult myocardial scarring in TAK patients indicates the utility of cardiac MRI with LGE for detecting myocardial changes [38].
Treatment
In case of suspicion or confirmation of vasculitis, it is necessary to refer the patient to a rheumatologist experienced in treating vasculitis for appropriate evaluation, treatment, and therapy monitoring. For patients with high-risk or complex anatomical anomalies requiring surgical intervention, such procedures should be performed at a high-volume center with experience in treating such patients, often necessitating a multidisciplinary approach involving cardiology, thoracic surgery, and rheumatology [16].
The treatment of patients with TAK involves two main approaches: the use of medications aimed at suppressing inflammation to reduce vascular damage, remodeling, and interventional procedures that can address the consequences of the disease, such as thrombus formation, tissue hypoperfusion, or ischemia [40].
For TAK, administering high doses of GCs at 40–60 mg per day is recommended to induce remission in the presence of activity. After achieving control of disease activity, it is recommended to taper the GC dose to the target dose (15–20 mg per day) over 2–3 months and, after one year, to ≤ 10 mg per day.
All patients with TAK should be prescribed non-biological disease-modifying antirheumatic drugs (DMARDs) in combination with GC. Tocilizumab or TNF-α inhibitors can be considered in case of relapse or refractory disease despite traditional DMARD therapy. In cases of severe relapse (with signs or symptoms of ischemia or progressive vasculitis), the restoration or increase of the GC dose is recommended [54].
As mentioned earlier, the involvement of coronary arteries in the pathological process significantly increases the mortality of patients with TAK. However, the optimal therapeutic strategy still needs to be established [60], and a standardized treatment algorithm for complex cases associated with CAL [23] currently needs to be developed.
The optimal treatment of CAL can significantly improve the prognosis of patients [13]. The treatment of patients with TAK with CAL remains complex and requires both medical therapy and interventional and surgical myocardial revascularization methods to prevent life-threatening events [40].
As reported by Pan et al. [61], the use of tocilizumab for 6 months significantly reduced disease activity in patients with TAK with CAL, allowed for a reduction in the dose of GCs, improved visualization results of coronary arteries, and reduced the total number of CAL from 23 to 15 with a decrease in vascular wall thickening. There are also data in the literature on the regression of severe stenosis in the ostium of both the left main trunk and the RCA after four months of treatment with GC and tocilizumab [62].
According to the study by Xu et al. [31], all 26 pediatric TAK patients were treated with GCs precombined with infliximab and MTX, TCZ and MTX, and cyclophosphamide (CP) in 17 (65.4%), 2 (7.7%), and 5 (19.2%) cases, respectively.
All patients received aspirin and/or warfarin. The median follow-up time was 4 years, and 24 patients were stable. Nine patients with coronary artery dilatation recovered in > 1 year, and 14 regressed in more than 0.5 years. None of the patients developed AMI or angina. Two patients died of irregular treatment during follow-up.
It is worth noting that the management of patients with TAK with CAL should be carried out according to the recommendations of the European Society of Cardiology, depending on the form of IHD [63–64].
The EULAR recommendations (2018) indicate that antiplatelet or anticoagulant therapy should not be routinely prescribed for treating patients with TAK unless indicated for other reasons (e.g., IHD, cerebrovascular disease).
In special situations, such as vascular ischemic complications or a high risk of CVD, these drugs may be considered on an individual basis [54]. However, Goel et al. [7] found that only ~50% of TAK patients requiring secondary CVD prevention received statins or antiplatelets within one year of study inclusion.
Endovascular and surgical interventions
The optimal strategy for myocardial revascularization in TAK with CAL has yet to be established [13, 65]. Huo et al. [23] noted that revascularization should be performed as quickly as possible in cases of severe vascular lesions. The treatment for such patients should be complemented with GCs, antiplatelet agents, nitrates, and statins.
Ideally, interventions should be performed during the inactive stage of TAK, as active inflammation contributes to restenosis [3, 13, 23], which remains a significant challenge [3, 13, 65]. However, in the EULAR recommendations for managing large vessel vasculitis (2018) [54], it is stated that elective endovascular interventions or reconstructive surgery should be performed during the phase of stable remission. However, arterial dissection or critical ischemia requires urgent referral to a vascular team [54].
As the authors note, in cases where coronary artery stenosis is severe and the lesion is unstable, early surgery should be considered even in the active period to prevent the development of CVE. However, GC therapy and immunosuppressive drugs should be administered concurrently [23].
In the 2021 recommendations from the American College of Rheumatology/Vasculitis Foundation for the Management of Giant Cell Arteritis and Takayasu Arteritis [66], it is mentioned that the decision regarding vascular surgery, the type, and timing of the intervention should be a joint decision between the vascular surgeon and the rheumatologist.
For patients with established TAK experiencing worsening signs of limb/organ ischemia during immunosuppressive therapy, there is a conditional recommendation to escalate immunosuppressive therapy instead of surgical intervention with an escalation of immunosuppressive therapy.
In another recommendation, it is stated that for patients with TAK and worsening signs of limb/organ ischemia, it is conditionally recommended to delay surgical intervention until the disease is quiescent rather than performing surgical intervention while the patient has active disease. If the patient has active disease, it is also recommended for patients undergoing surgical intervention for TAK to use high doses of GCs conditionally in the peri-procedural period.
Research data have shown that CABG surpasses endovascular intervention, primarily because restenosis is more frequently encountered with the latter method of myocardial revascularization [13, 23, 65].
When comparing long-term treatment outcomes in patients with TAK with CAL using CABG and PCI with stenting, the risk of major adverse cardiac events (MACE; MI, repeat revascularization, cardiac death) was significantly higher in the group of patients undergoing PCI with stenting than CABG during a median follow-up period of 41 months (p < 0.001), especially in those who underwent revascularization in the active stage of TAK (p = 0.001).
In contrast, no significant differences were observed in patients who underwent revascularization in the remission stage (p = 0.138). Moreover, in patients receiving prednisone in the active stage of the disease, the risk of MACE was significantly lower than in those who did not use it (p = 0.028). The prognostic predictors of MACE in patients with TAK and CAL were revascularization strategy (PCI/CABG: HR = 9.854, 95% CI: 2.183–44.472; p = 0.003) and active inflammation (HR = 10.577, 95% CI: 2.351–47.582; p = 0.002) [13].
Another study demonstrated that over a median follow-up period of 101 months, patients undergoing PCI with stenting had a significantly higher incidence of restenosis than patients undergoing CABG (63.2% vs. 25%). It is worth noting that both groups had a low rate of GC usage (21.1% vs. 16.7%). The authors concluded that PCI with stenting had a very high stent restenosis rate in patients without GCs, making CABG a potentially better treatment option [65].
In a multicenter study, 37.2% of patients with CAL received conservative treatment, 10.6% underwent percutaneous coronary angioplasty, 29.7% underwent PCI with stent implantation, and 22.3% underwent surgical treatment. The authors noted that PCI with stent implantation was a safe and effective intervention with a high success rate, but the stent restenosis rate was high. On the other hand, CABG showed a stable curative effect [23].
A meta-analysis of 2 studies showed that restenosis occurred more frequently with endovascular intervention than with open surgical intervention for CAL (OR = 7.38, 95% CI: 2.36–23.10; p = 0.001). However, in a meta-analysis of 16 studies, there was no significant difference in restenosis in the coronary arteries between the two groups (OR = 3.27, 95% CI: 0.80–13.31; p = 0.10) [67].
Yuan et al. [3] also found that interventional procedures were associated with a higher frequency of re-intervention than surgical treatment, which is a better choice for revascularization than interventional therapy, although early GC therapy and treatment with MTX or CP should be considered. However, Zhang et al. [41] concluded that PCI is effective in MI and TAK, and timely immunosuppressive therapy can improve long-term results.
According to the study data, over a median follow-up period of 6 years, the overall success rate was 79% for open surgery procedures (average graft patency 9.4 years) and 52% for endovascular procedures (p = 0.035). Procedural failure was significantly reduced in patients receiving preoperative immunosuppressive therapy, particularly for endovascular procedures (p = 0.001).
A thorough preoperative assessment can improve vascular intervention outcomes in patients with TAK, including evaluating disease activity and providing optimal immunomodulatory therapy before and after the procedure [68]. Koster et al. [16] emphasized that continuous vasculitis treatment with immunosuppressive therapy is crucial regardless of whether endovascular or surgical revascularization is planned.
It is important to note that when using drug-eluting stents (DES), the immunosuppressive agents contained in such stents can suppress vascular autoimmune inflammatory responses, reducing the risk of stent restenosis [23].
Yokota et al. [69] point out that since stent restenosis can result from a complex combination of neointimal proliferation and autoimmune mechanisms, physicians should consider performing revascularization in combination with GC therapy for treating CAD in TAK, which has proven effective in preventing restenosis.
It should be noted that according to the study by Huang et al. [60], there was no significant difference in cardiovascular death between medical treatment and revascularization (5.1% vs. 9.8%; p = 0.971). Subgroup analysis showed that the mortality caused by CVD was also similar between CABG and PCI (7.1% vs. 13.0%; p = 0.772).
However, restenosis was more frequently observed in PCI than CABG (39.3% vs. 8.7%; p = 0.022, respectively). In contrast, conflicting data were obtained by Huo et al. [23], who found that when analyzing survival using the Kaplan-Meier method, the mortality rate in the conservative treatment group was significantly higher (37.1%) than in the intervention therapy groups (26.3%) and surgical treatment in patients with myocardial ischemia and neurological symptoms.
Prognosis
According to the study by Goel et al. [7], all-cause mortality was higher in patients with TAK (adjusted HR = 1.88, 95% CI: 1.29–2.76) compared to age- and sex-matched individuals, and CVD was the cause of death in 45.3% of patients with TAK [9].
Ci et al. [6] reported that factors such as active disease at baseline, male sex, and PCI were associated with a worse long-term prognosis for patients with TAK and coronary artery stenosis. Other researchers found that among patients with TAK with CAL, men had a higher risk of long-term mortality than women. Over seven years, the overall mortality was higher in men than in women (21.4% vs. 1.5%; p = 0.003). Lei et al. [24] observed that the event-free survival rate in patients with TAK and CAL in the pediatric group was 55.6% after 60 months of observation, while in the adult group it was 22.7%.
Major adverse cardiac events (MACE), defined as cardiac death, non-fatal (MI), rehospitalization due to angina or HI, and repeated coronary artery revascularization, were observed in 12.8% of patients with TAK and CAL during one year of observation.
It was also noted that MACE was nearly 44% higher in men with TAK and CAL than in women [32]. However, Xu et al. [31] found that after treatment, there was no significant difference in the survival rate in children with CAL compared to those without CAL (p > 0.05).
Conclusions
Thus, TAK is a more common cause of CAL development, especially in young patients, than previously thought, and cardiovascular death due to CAL is not rare. The presence of nonspecific systemic symptoms accompanied by the absence of a pulse, different blood pressure, or symptoms indicative of ischemia requires investigation with visualization studies.
In TAK, the ostia and proximal segments of coronary arteries are more frequently affected, leading to stenosis or occlusion with the development of stable angina, unstable angina, and MI. Early diagnosis of TAK and CAL, especially occlusion or hemodynamically significant stenosis, and early initiation of appropriate treatment prevent the development of dangerous complications of this disease, reduce mortality, and improve the prognosis of patients.
Further research is needed to optimize the management of patients with TAK and CAL to achieve better long-term outcomes, establish prognostic factors, and implement preventive measures for severe ischemic events in patients with TAK.