Introduction
Overlap syndromes (OS) are systemic autoimmune diseases in which diagnostic criteria of at least two or more connective tissue diseases (CTDs) are fulfilled [1]. The most usual associations include systemic sclerosis (SSc), rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), dermatomyositis (DM) or polymyositis (PM), and primary Sjögren’s syndrome (pSS) [1]. Synovitis and myositis were reported to be more common in patients with scleroderma overlap syndrome (SSc-OS) than in patients with SSc [2]. Also, they have a smaller percentage of digital ulcers (DU) compared to SSc patients [1]. Although both classes had the same rates of interstitial lung disease (ILD), pulmonary arterial hypertension (PAH) was reported to be less common in SSc-OS [2].
It was found that patients with OS usually have milder clinical symptoms than patients with any of the CTDs [3]. In patients with SSc-OS, it was found that the prevalence of OS was significantly superior in patients with limited cutaneous SSc (LcSSc) than those with diffuse cutaneous SSc (dcSSc) [1].
Nailfold capillaroscopy is a simple, noninvasive, inexpensive imaging method and a practical instrument for assessment of microvasculature in systemic CTDs such as SSc, SLE, mixed connective tissue disease (MCTD), and DM [4]. It was reported that in patients with SSc there were associations between the late scleroderma pattern of capillaroscopy and disease activity, extent of skin involvement, DU, calcinosis, ILD, and PAH [5]. In addition, other studies showed some relationships between cardiopulmonary complications in patients with SSc and presence of capillary loss in capillaroscopy [6–8].
In one study, scleroderma pattern of capillaroscopy was observed in 94% of patients with SSc and 46% of patients with OS [9]. In another study, there was no significant difference in the reduction of capillary density and prevalence of scleroderma pattern among SSc patients with or without overlap, and the reduction of capillary density was more common in SSc and SSc-OS than in the control group [10].
In this study, we evaluated the clinical, laboratory, and capillary findings of patients with SSc-OS and their subgroups in comparison with patients with LcSSc; also, we followed the patients and compared their complications in the follow-up.
Material and methods
Material
In this study, we evaluated 135 patients aged > 17 years who were referred to clinics of rheumatology affiliated to Shiraz University of Medical Sciences or the capillaroscopy clinic of Hafez between September 2010 and September 2020.
They fulfilled the criteria of SSc-OS and were divided into subgroups [11]. Patients with more than one CTD were classified as OS patients (these CTDs were SSc, SLE, RA, inflammatory myositis and pSS) [1]. Patients with SSc-OS had the 2013 American College of Rheumatology/ European Alliance of Associations for Rheumatology (ACR/EULAR) SSc diagnostic criteria [12] along with another systemic CTD such as inflammatory myositis [13], RA [14], SLE [15], or pSS [16]. The control group consisted of patients with LcSSc based on the 2013 ACR/EULAR scleroderma classification criteria with the same skin score of patients with SSc-OS. Rheumatoid arthritis and pSS were diagnosed according to the new ACR/EULAR classification criteria [14, 16], and SLE was diagnosed according to the 2019 score [15]. Patients with DM/PM who met the criteria of Bohan and Peter for definite and probable DM/PM [13, 17] were also included.
Patients with LcSSc or SSc-OS who were smokers, had diabetes, had an MCTD [18] which fulfilled the criteria of Kohn et al. and those with unavailable capillaroscopy were excluded from the study. Patients with concomitant antiphospholipid syndrome, primary biliary cirrhosis, or autoimmune hepatitis were not diagnosed with OS.
Data collection
The documents and reports of all patients and controls during the study who were referred for doing their routine visit and capillaroscopy with available history, examination, skin score, and laboratory tests on the day of capillaroscopy were reviewed; the researchers obtained the participants’ informed consent. Then, in patients with follow-up capillaroscopy, the progression of involvement of major organs including pulmonary involvement, skin score, PAH and DU, and changes in capillaroscopic and laboratory findings and medications used by the patient from documents of patients referred to the scleroderma and capillaroscopy clinic were extracted over the years and recorded in a questionnaire.
Patients’ characteristics and clinical assessment
All patients were evaluated for age, sex, duration of disease and serology (antinuclear antibody [ANA], anti-double-stranded DNA antibody [anti-dsDNA], anti-Jo1, anticentromere antibody [ACA], anti-SCL70, anti-La, anti-Ro and anti-Smith [anti-SM]), anticardiolipin anti-body (ACLA), and anti-cyclic citrullinated peptide antibody (ACPA) by the enzyme-linked immunosorbent assay (ELISA) method and complement. Erythrocyte sedimentation rate (ESR) was checked by the Westergren method. Serum C-reactive protein (CRP) was checked by the nephelometric method and rheumatoid factor (RF) by the latex test.
Clinical findings such as limited or diffuse skin involvement and skin score in patients with SSc were assessed according to the definition of Khanna et al. based on the modified Rodnan skin score (MRSS score) [19, 20], and the presence or absence of Raynaud’s phenomenon (RP) was recorded. Gastrointestinal (GI) symptoms, including dysphagia, heart burn, difficult swallowing, feeling of being full, constipation and diarrhea were reported too.
Pulmonary involvement was assessed by baseline high resolution CT (HRCT) scan at the first screening, in all patients. We assessed the pulmonary involvement based on symptoms, examination, pulmonary function tests and HRCT scan, although to be included in the study our patients needed to have HRCT scan documented pulmonary involvement based on the pulmonologist recommendation, which was divided into < 20% involvement and > 20% involvement of the lung.
Cardiac involvement was defined as: PAH (when systolic pulmonary arterial pressure (sPAP) > 40 mmHg was detected in Doppler echocardiography) [20] or presence of any pericardial, valvular or myocardial involvement in echocardiography. Due to the invasiveness of right heart catheterization and not having the agreement of all patients for doing it, traditional echocardiogram-estimated right ventricular systolic pressure values > 40 mmHg were used to categorize suspected PAH [21–23].
On the same day, the patients underwent capillaroscopy by Euromex ST.1740 stereomicroscope (Netherlands) at × 250 power and a Cmex D.C.5000 video camera (5 megapixels).
Immersion oil was applied on the nailfold bed to improve resolution.
Eight fingers of the two hands excluding the thumbs were assessed. The fingers with thick nailfolds and ulcerated ones were not studied. The data were recorded in a form including the shape of the capillaries (normal or abnormal shape); capillary diameter, i.e. the largest diameter of the apical side of the nailfold capillaries (if it was ≥ 20 µm, it was marked as capillary dilatation and if it was ≥ 50 µm, it was marked as giant loop); capillary length (the mean of the 3 greatest lengths ≥ 300 µm was called elongated); mean capillary density (the mean capillary density in 1 mm > 9 capillaries/mm = very good density, between 7 and 9 capillaries/mm = good density, between 4 and 6 capillaries/mm = reduced (low) density, < 4 capillaries/mm = very low density); and presence or absence of hemorrhage (the total number) in the involved fingers based on the international Delphi consensus for reporting the data and the PANLAR capillaroscopy study group consensus. The whole nailfold capillaroscopy findings were defined as follows: normal, scleroderma pattern (classified as early, active, and late scleroderma pattern), or non-specific abnormalities [24, 25].
Statistical analysis
According to the study of Lambova et al. [26] with a power test α = 0.05, 76 patients was considered as the sample size for each group. Data were analyzed through SPSS-22 using the κ-square test to examine the changes. The results were shown as mean ±standard deviation. P-value < 0.05 was considered statistically significant. Descriptive statistics included mean, standard deviation, frequency and percentage of frequency, and inferential statistics included the χ2 test, independent t-test, paired sample t-test, and Mann-Whitney test.
Bioethical standards
Ethics approval and consent to participate in the study were approved by the Human Ethics Committee of our University of Medical Science with the code number of IR.SUMS.MED.REC.1399.189. Informed consent was obtained from all the participants before entering the study. There was no animal experiment in this study.
Results
In total, 135 patients (70 [51.9%] with SSc-OS and 65 [48.1%] with lcSSc with the same skin score [the mean Rodnan Skin Score in the SSc-OS and LcSSc groups was 4.3 and 6.55, respectively, p = 0.053]) were included in the study; of them, 127 (94.1%) patients were female and 8 (5.9%) were male. Of the total number of subjects studied, 65 (48.1%) had LcSSc and 70 (51.9%) had SSc-OS. Out of 70 patients with SSc-OS, 9 (12.9%) had SSc-myositis, 8 (11.43%) with SSc-DM and 1 (1.43%) with SSc-PM, 11 (15.7%) had SSc-RA, 5 (7.1%) had SSc-SLE, and 45 (64.3%) had SSc-pSS.
There was no significant difference between the mean age and sex of both groups (p = 0.525 and 0.481, respectively); also, there was no significant difference in the mean age among the subgroups of SSc-OS (p = 0.525) (Table I).
Table I
Demographic data, disease information, and laboratory variables in SSc-OS subgroup patients and LcSSc ones
| Variable | All patients (n = 135) | LcSSc (n = 65) | SSc-OS syndrome (n = 70) | P-value* | SSc-RA (n = ID | SSc-SLE (n = 5) | SSc-pSS (n = 45) | SSc-myositis (n = 9) | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||
| Male [n (%)] | 8 (5.9) | 5 (7.7) | 3 (4.3) | 0.481 | 0(0) | 0 (0%) | 1 (2.2) | 2 (22.2) | 0.080 |
| Female [n (%)] | 127 (94.1) | 60 (92.3) | 67 (95.7) | 11 (100) | 5 (100%) | 44 (97.8) | 7 (77.8) | ||
| Age [years] mean ± SD | 49.40 ±11.8 | 49.70 ±11.97 | 49.28 ±11.78 | 0.525 | 48.09 ±14.72 | 39.80 ±8.25 | 52.28 ±9.84 | 41.00 ±13.18 | 0.525 |
| Disease Duration [years] (mean ±SD) | 12.55 ±7.90 | 14.92 ±7.17 | 10.35 ±7.96 | 0.738 | 8.90 ±6.50 | 7.00 ±3.16 | 10.91 ±8.34 | 11.22 ±9.65 | 0.674 |
| RP Duration [months] (mean ±SD) | 54.46 ±8.64 | 66.29 | 56.39 | 0.122 | 45.18 ±16.40 | 45.00 ±21.56 | 77.77 ±16.20 | 91.37 ±44.57 | 0.836 |
| ANA | |||||||||
| Checked [n (%)] | 116 (85.9) | 52 (80) | 64 (91.4) | 0.323 | 10 (90.9) | 5(100) | 43 (95.6) | 6 (66.7) | 0.064 |
| Positive [n (%)] | 102 (75.6) | 44 (67.9) | 58 (82.8) | 7 (63.6) | 5(100) | 41 (91.1) | 5 (55.6) | ||
| dsDNA | |||||||||
| Checked [n (%)] | 77 (57) | 30 (46.2) | 47 (67.1) | 0.237 | 9 (81.8) | 5(100) | 31 (68.9) | 2 (22.2) | 0.001 |
| Positive [n (%)] | 7 (5.2) | 1 (1.5) | 6 (8.5) | 0(0) | 4(80) | 2 (4.4) | 0(0) | ||
| RF | |||||||||
| Checked [n (%)] | 73 (54.1) | 29 (44.6) | 44 (62.9) | 0.000 | 11 (100) | 0(0) | 30 (66.7) | 3 (33.3) | 0.000 |
| Positive [n (%)] | 31 (23) | 5 (7.6) | 26 (37.1) | 11 (100) | 0(0) | 15 (33.3) | 0(0) | ||
| C3 | |||||||||
| Checked [n (%)] | 60 (44.4) | 22 (33.8) | 38 (54.3) | 0.176 | 5 (45.5) | 5(100) | 26 (57.8) | 2 (22.2) | 0.307 |
| Low [n (%)] | 3 (2.2) | 0(0) | 3 (4.2) | 1 (9.1) | 1(20) | 1 (2.2) | 0(0) | ||
| C4 | |||||||||
| Checked [n (%)] | 60 (44.4) | 22 (33.8) | 38 (54.3) | 0.115 | 5 (45.5) | 5(100) | 26 (57.8) | 2 (22.2) | 0.445 |
| Low [n (%)] | 4(3) | 0(0) | 4 (5.7) | 1 (9.1) | 1(20) | 2 (4.4) | 0(0) | ||
| SCL70 | |||||||||
| Checked [n (%)] | 102 (75.6) | 42 (64.6) | 60 (85.7) | 0.000 | 7 (63.6) | 5(100) | 41 (91.1) | 7 (77.8) | 0.473 |
| Positive [n (%)] | 34 (25.2) | 24 (36.9) | 10 (14.2) | 1 (9.1) | 2(40) | 6 (13.3) | 1 (11.1) | ||
| ACA | |||||||||
| Checked [n (%)] | 55 (40.7) | 15 (23.1) | 40 (57.1) | 0.854 | 6 (54.5) | 4(80) | 25 (55.6) | 5 (55.6) | 0.097 |
| Positive [n (%)] | 25 (18.5) | 7 (10.7) | 18 (25.7) | 2 (18.1) | 0(0) | 15 (33.3) | 1 (11.1) | ||
| Anti-RO/SSA | |||||||||
| Checked [n (%)] | 102 (75.6) | 41 (63.1) | 61 (87.1) | 0.000 | 8 (72.7) | 5(100) | 41 (91.1) | 7 (77.8) | 0.000 |
| Positive [n (%)] | 45 (33.3) | 4 (6.1) | 41 (58.5) | 4 (36.3) | 4(80) | 33 (73.3) | 0(0) | ||
| Anti-LA/SSB | |||||||||
| Checked [n (%)] | 102 (75.6) | 41 (63.1) | 61 (87.1) | 0.237 | 8 (72.7) | 5(100) | 41 (91.1) | 7 (77.8) | 0.695 |
| Positive [n (%)] | 7 (5.2) | 1 (1.5) | 6 (8.5) | 0(0) | 0(0) | 6 (13.3) | 0(0) | ||
| Anti-Sm | |||||||||
| Checked [n (%)] | 101 (74.8) | 41 (63.1) | 60 (85.7) | 0.269 | 7 (63.6) | 5(100) | 41 (91.1) | 7 (77.8) | 0.006 |
| Positive [n (%)] | 3 (2.2) | 0(0) | 3 (4.2) | 0(0) | 2(40) | 0(0) | 1 (11.1) | ||
| Jo-1 | |||||||||
| Checked [n (%)] | 101 (74.8) | 41 (63.1) | 60 (85.7) | 0.513 | 7 (63.6) | 5(100) | 41 (91.1) | 7 (77.8) | 0.537 |
| Positive [n (%)] | 2 (1.5) | 0(0) | 2 (2.8) | 0(0) | 0(0) | 1 (2.2) | 1 (11.1) | ||
| ACPA | |||||||||
| Checked [n (%)] | 53 (39.3) | 16 (24.6) | 37 (52.9) | 0.182 | 11 (100) | 1(20) | 22 (48.9) | 3 (33.3) | 0.000 |
| Positive [n (%)] | 14 (10.4) | 2 (3.1) | 12 (17.1) | 9 (81.8) | 1(20) | 2 (4.4) | 0(0) | ||
| ACLA | |||||||||
| Checked [n (%)] | 34 (25.2) | 17 (26.2) | 17 (24.3) | 0.485 | 1 (9.1) | 4(80) | 11 (24.4) | 1 (11.1) | 0.110 |
| Positive [n (%)] | 2 (1.5) | 0(0) | 2 (2.8) | 0(0) | 2(40) | 0(0) | 0(0) | ||
| CRP | |||||||||
| Checked [n (%)] | 135 (100) | 65 (100) | 70 (100) | 0.429 | 11 (100) | 11 (100) | 45 (100) | 9 (100) | 0.621 |
| Positive ≥6 [n (%)] | 20 (14.8) | 8 (12.3) | 12 (17.1) | 3 (27.3) | 0(0) | 7 (15.6) | 2 (22.2) | ||
| ESR | |||||||||
| Checked [n (%)] | 135 (100) | 65 (100) | 70 (100) | 0.330 | 11 (100) | 11 (100) | 45 (100) | 9 (100) | 0.667 |
| Positive ≥ 25 [n (%)] | 32 (23.7) | 13 (20) | 19 (27.1) | 3 (27.3) | 1(20) | 11 (24.4) | 4 (44.4) | ||
* P-values comparing the difference between sex, age, disease duration, RP disease duration and lab test frequency between the two groups of patients including SSc overlap syndrome patients and LcSSc patients (p-value*) were considered significant if < 0.05.
P-values comparing the difference between sex, age, disease duration, RP disease duration, and lab test frequency between the subgroups of SSc overlap syndrome patients (p-value) were considered significant if < 0.05.
AC – anticentromere antibody, ACLA – anticardiolipin antibody, ACPA – anti-cyclic citrullinated peptide antibody, ANA – antinuclear antibody, anti-dsDNA – anti-double-stranded DNA antibody, anti-SM – anti-smooth, C – complement, CRP – C-reactive protein, ESR – erythrocyte sedimentation rate, OS – overlap syndrome, RA – rheumatoid arthritis, RF – rheumatoid factor, RP – Raynaud’s phenomenon, SD – standard deviation, SLE – systemic lupus erythematosus, SSc – systemic sclerosis.
History of RP was significantly lower in the SSc-OS group (p = 0.007) (Table II). There were no significant differences between RP duration at the onset of the disease in both groups (p = 0.122) (Table I).
Table II
Frequency of clinical manifestation and mediation usage in patients with LcSSc and SSc-OS
| Variable | Total | SSc-OS | LcSSc | P-value* | |||
|---|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | N | n (%) | ||
| Skin score | |||||||
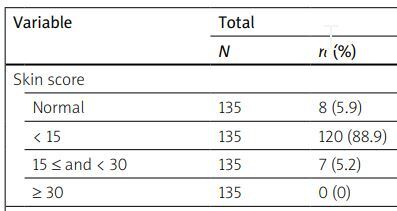
| Normal | 135 | 8 (5.9) | 70 | 7 (10) | 65 | 1 (1.5) | 0.053 |
| < 15 | 135 | 120 (88.9) | 70 | 61 (87.1) | 65 | 59 (90.8) | |
| 15 ≤ and < 30 | 135 | 7 (5.2) | 70 | 2 (2.9) | 65 | 5 (7.7) | |
| ≥ 30 | 135 | 0 (0) | 70 | 0 (0) | 65 | 0 (0) | |
| Telangiectasia | 135 | 74 (54.8) | 70 | 34 (48.6) | 65 | 40 (61.5) | 0.130 |
| RP needing vasodilator | 135 | 121 (89.6) | 70 | 58 (82.9) | 65 | 63 (96.9) | 0.007 |
| Pitting scar | 135 | 41 (30.4) | 70 | 7 (10) | 65 | 34 (52.3) | 0.000 |
| Digital tip ulcer | 135 | 25 (18.5) | 70 | 5 (7.1) | 65 | 20 (30.8) | 0.000 |
| Digital gangrene | 135 | 2 (1.5) | 70 | 0 (0) | 65 | 2 (3.1) | 0.230 |
| Limitation of FTP | 135 | 8 (5.9) | 70 | 2 (2.9) | 65 | 6 (9.2) | 0.262 |
| Arthritis | 135 | 14 (10.4) | 70 | 11 (15.7) | 65 | 3 (4.6) | 0.035 |
| Proximal muscle weakness | 135 | 7 (5.2) | 70 | 7 (10) | 65 | 0 (0) | 0.014 |
| GI abnormality | 135 | 87 (64.4) | 70 | 37 (52.9) | 65 | 50 (76.9) | 0.004 |
| Dysphagia | 135 | 31 (23) | 70 | 6 (8.6) | 65 | 25 (38.5) | 0.000 |
| Heart burn | 135 | 82 (60.7) | 70 | 36 (51.4) | 65 | 46 (70.8) | 0.021 |
| Difficult swallowing | 135 | 20 (14.8) | 70 | 1 (1.4) | 65 | 19 (29.2) | 0.000 |
| The feeling of being full | 135 | 16 (11.9) | 70 | 2 (2.9) | 65 | 14 (21.5) | 0.001 |
| Diarrhea | 135 | 9 (6.7) | 70 | 1 (1.4) | 65 | 8 (12.3) | 0.014 |
| Constipation | 135 | 4 (3) | 70 | 0 (0) | 65 | 4 (6.2) | 0.051 |
| Lung involvement | 135 | 46 (34.1) | 70 | 19 (27.1) | 65 | 27 (41.5) | 0.078 |
| Pulmonary hypertension | 135 | 6 (4.4) | 70 | 3 (4.3) | 65 | 3 (4.6) | 1.000 |
| Dyspnea with walking | 135 | 37 (27.4) | 70 | 14 (20) | 65 | 23 (35.4) | 0.045 |
| Basilar rales | 135 | 23 (17) | 70 | 8 (11.4) | 65 | 15 (23.1) | 0.072 |
| HRCT involvement* | 135 | 46 (34.1) | 70 | 19 (27.1) | 65 | 27 (41.5) | 0.078 |
| HRCT involvement | |||||||
| > 20% | 135 | 43 (31.9) | 70 | 25 (35.7) | 65 | 18 (27.7) | 1.000 |
| < 20% | 135 | 3 (2.2) | 70 | 2 (2.9) | 65 | 1 (1.5) | |
| Heart problem | 135 | 9 (6.7) | 70 | 3 (4.3) | 65 | 6 (9.2) | 0.312 |
| Hypertension | 135 | 7 (5.2) | 70 | 6 (8.6) | 65 | 1 (1.5) | 0.117 |
| Renal failure | 135 | 0 (0) | 70 | 0 (0) | 65 | 0 (0) | - |
| Immunosuppressive administered | 135 | 40 (29.6) | 70 | 14 (20) | 65 | 26 (40) | 0.028 |
In patients with SSc-OS, the prevalence of DU and GI symptoms was significantly lower (p = 0.000 and 0.004, respectively), but cardiac involvement, pulmonary hypertension and fingertip-to-palm (FTP) distance, prevalence of ILD, and severity of lung involvement were not different between the two groups (p > 0.05). Arthritis was significantly more frequent in the SSc-OS group (p = 0.035) (Table II).
The rate of positivity of ANA, dsDNA, ACA, anti-LA, anti-Sm, anti-Jo1, ACLA, ACPA, CRP, ESR, low C3, and low C4 was not significantly different between the two groups (p > 0.05), but that of SCL70 was higher in the LcSSc group (p = 0.000); also, the rate of positivity of RF and anti-RO was higher in the SSc-OS group (p = 0.000 and p = 0.000, respectively).
The rates of positivity of ANA, SCL70, ACA, anti-LA, anti-Jo1, ACLA, CRP, ESR, low C3, and low C4 were not significantly different between the subgroups (p > 0.05), but those of dsDNA and anti-Sm were higher in the SSc-SLE group (p = 0.001 and p = 0.006, respectively); the rate of positivity of anti-RO was higher in the SSc-SLE and SSc-pSS groups (p = 0.000) and that of RF and ACPA was higher in the SSc-RA group (p = 0.000 and p = 0.000, respectively).
The demographic data, disease specifications, and laboratory variables in SSc-OS subgroups and LcSSc patients and means are presented in Table I.
Among patients with SSc-OS, 14 (20%) used immunosuppressant drugs such as azathioprine (AZA), cyclophosphamide (CFM), mycophenolate mofetil (MMF), and tacrolimus, but in the LcSSc group, 26 (40%) patients used immunosuppressant drugs (AZA, CFM and MMF); therefore, the number of patients taking immunosuppressive drugs was lower in the SSc-OS group (p = 0.028) (Table II).
In capillaroscopy, the non-specific pattern was higher and the scleroderma pattern was lower in the SSc-OS group than those with LcSSc (p = 0.000); also, avascular area was significantly lower in the SSc-OS group (p = 0.018), but dilated capillary, giant capillary, reduced capillary density, abnormal capillary morphology, presence of hemorrhage and elongated capillary were not significantly different between the two groups (p > 0.05); further, there was no significant difference between the subgroups in scleroderma patterns in capillaroscopy (p = 0.207) (Table III). No scleroderma-like pattern was seen in capillaroscopy of our patients.
Table III
Capillaroscopic manifestations in patients with LcSSc, SSc-OS, and its subgroups
| Variable | All patients (n = 135) n (%) | LcSSc (n = 65) n (%) | SSc-OS (n = 70) n (%) | P-value* | SSc RA (n = 11) n (%) | SSc SLE (n = 5) n (%) | SSc pSS (n = 45) n (%) | SSc Myositis (n = 9) n (%) | P-value |
|---|---|---|---|---|---|---|---|---|---|
| Scleroderma pattern | 99 (73.3) | 58 (89.2) | 41 (58.5) | 0.000* | 8 (72.7) | 4(80) | 23 (51.1) | 6 (66.6) | 0.207 |
| Scleroderma pattern | |||||||||
| “Early” | 35 (25.9) | 19 (29.2) | 16 (22.9) | 3 (27.3) | 1(20) | 11 (24.4) | 1 (11.1) | ||
| “Active” | 53 (39.3) | 30 (46.2) | 23 (32.9) | 5 (45.5) | 2(40) | 12 (26.7) | 4 (44.4) | ||
| “Late” | 11 (8.1) | 9 (13.8) | 2 (2.9) | 0(0) | 1(20) | 0(0) | 1 (11.1) | ||
| “Nonspecific” pattern | 36 (26.7) | 7 (10.8) | 29 (41.5) | 3 (27.3) | 1(20) | 22 (48.9) | 3 (33.3) | ||
| Dimension | |||||||||
| Presence of dilated capillaries | 0.007 | ||||||||
| < 33% | 33 (24.5) | 12 (18.5) | 21 (30) | 0.133 | 0(0) | 1(20) | 15 (33.3) | 5 (55.5) | |
| 33-66% | 97 (72) | 51 (78.5) | 46 (66) | 11 (100) | 3(60) | 28 (62.2) | 4 (44.4) | ||
| > 66% | 1 (0.74) | 0(0) | 1 (1.4) | 0(0) | 1(20) | 0(0) | 0(0) | ||
| Presence of giant capillaries | 1.000 | ||||||||
| < 33% | 58 (43) | 30 (46.1) | 28 (40) | 0.295 | 6 (54.5) | 3(60) | 15 (33.3) | 4 (44.4) | |
| 33-66% | 35 (25.9) | 22 (33.8) | 13 (18.5) | 2 (18.1) | 1(20) | 8 (17.7) | 2 (22.2) | ||
| > 66% | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | 0(0) | ||
| Reduced capillary density < 7/mm | 0.230 | ||||||||
| 4-6/mm | 64 (47.4) | 35 (53.8) | 29 (41.4) | 0.088 | 5 (45.5) | 3(60) | 15 (33.3) | 6 (66.7) | |
| < 4/mm | 6 (4.4) | 5 (7.7) | 1 (1.4) | 0(0) | 0(0) | 0(0) | 1 (11.1) | ||
| Presence of abnormal capillary morphology | 0.039 | ||||||||
| < 33% | 63 (46.7) | 27 (41) | 36 (51.4) | 0.067 | 9 (81.8) | 0(0) | 22 (48.9) | 5 (55.5) | |
| 33-66% | 11 (8.1) | 4 (6.1) | 7(10) | 1 (9.1) | 1(20) | 2 (4.4) | 3 (33.3) | ||
| > 66% | 4(3) | 4 (6.1) | 0(0) | 0(0) | 0(0) | 0(0) | |||
| Avascular areas > 0.500 in distal row | 0.574 | ||||||||
| 1 or 2 | 27 (20) | 12 (18.5) | 15 (21.5) | 0.018* | 4 (36.4) | 1(20) | 8 (17.7) | 2 (22.2) | |
| > 2 | 17 (12.6) | 14 (21.6) | 3 (4.3) | 0(0) | 0(0) | 2 (4.4) | 1 (11.1) | ||
| Extensive | 1 (0.74) | 0(0) | 1 (1.4) | 0(0) | 0(0) | 0(0) | 1 (11.1) | ||
| Presence of hemorrhage | 88 (65.2) | 46 (70.8) | 42 (60) | 0.189 | 9 (81.8) | 2(40) | 25 (55.6) | 6 (66.7) | 0.344 |
| Elongated capillary | 0.784 | ||||||||
| 00-500 urn | 36 (26.7) | 14 (21.5) | 22 (31.4) | 0.127 | 4 (36.4) | 2(40) | 14 (31.1) | 2 (22.2) | |
| > 500 urn | 2 (1.5) | 0(0) | 2 (2.9) | 0(0) | 0(0) | 1 (2.2) | 1 (11.1) | ||
* P-values comparing the difference between capillaroscopy variables’ frequency between the two groups of patients including SSc-OS, and LcSSc (p-value*) were considered significant if < 0.05.
There was no significant difference between organ involvement (lung, DU, PAH, abnormal skin score and arthritis) and capillaroscopic patterns in the two groups (p > 0.05) (Table IV).
Table IV
Association between organ involvement and scleroderma patterns of capillaroscopy in SSc-OS and LcSSc groups
| Pattern of capillaroscopy | DU | Lung involvement | PAH | Abnormal skin score | Arthritis |
|---|---|---|---|---|---|
| SSc-OS (n = 70) | |||||
| Scleroderma pattern [n (%)] | 4 (80) | 12 (63.2) | 1 (33.3) | 36 (57.1) | 6 (54.5) |
| Non specific [n (%)] | 1 (20) | 7 (36.8) | 2 (66.7) | 27 (42.9) | 5 (45.5) |
| Total [n (%)] | 5 (7.1) | 19 (27.1) | 3 (4.3) | 63 (90) | 11 (15.7) |
| P-value* | 0.395 | 0.634 | 0.566 | 0.191 | 1.000 |
| LcSSc (n = 65) | |||||
| Scleroderma pattern [n (%)] | 18 (90) | 23 (85.2) | 2 (66.7) | 57 (89.1) | 3 (100) |
| Non specific [n (%)] | 2 (10) | 4 (14.8) | 1 (33.3) | 7 (10.9) | 0(0) |
| Total [n (%)] | 20 (30.8) | 27 (41.5) | 3 (4.6) | 64 (98.5) | 3 (4.6) |
| P-value* | 0.894 | 0.437 | 0.294 | 0.671 | 1.000 |
* P-values comparing the difference between the organ involvement with scleroderma patterns of capillaroscopy between the two groups of patients including SSc-OS syndrome patients and LcSSc patients (p-value*) were considered significant if < 0.05. No significant relationship was found between capillaroscopy patterns and organ involvement in two groups.
Among the subgroups, evidence of ILD in HRCT scan was significantly higher in the SSc-SLE than the others (p = 0.048), and arthritis was significantly higher in the SSc-RA overlap than the other subgroups (p = 0.000), but in PAH, DU, and abnormal FTP, there was no significant difference between subgroups (p > 0.05).
Thirty-five patients from the SSc-OS group and 65 patients from the SSc group were referred for follow-up with a mean follow-up duration of 3.71 ±2.63 years in SSc-OS and 3.22 ±1.87 years in LcSSc with no significant difference (p = 0.366). The patients were referred from different clinics and physicians so none of the included patients had follow-up for capillaroscopy.
In the SSc-OS group, from 26 patients without ILD at the first visit, 5 (19.2%) patients, and in the LcSSc group, from 38 patients without ILD, 13 (34.2%) showed evidence of ILD in the follow-up, so progression of lung involvement was significantly lower in the SSc-OS group (p = 0.000).
In the SSc-OS group, from 32 patients without DU in the first visit, no patient showed evidence of DU in the follow-up, but in the LcSSc group, from 45 patients without DU, in 7 (15.6%) patients DU was seen, so new DU was not seen in the SSc-OS group and was significantly higher in the LcSSc group (p = 0.000). In the SSc-OS group, from 5 patients who had a normal skin score at the first visit, no patient showed an increase in skin score in the follow-up; also, from 29 patients who had a mild skin score, 1 (3.4%) showed a moderate skin score (p = 0.000). Moreover, in the LcSSc group, the only patient who had a normal skin score at the first visit had a mild skin score at the follow-up. Also, from 59 patients who had a mild skin score, 3 (5.1%) showed a moderate skin score (p = 0.003), so the progression of skin score was significantly lower in the SSc-OS group than in the LcSSc patients.
In the SSc-OS group, from 34 patients without heart involvement at the first visit, only 1 (2.9%) patient (p = 0.057), and in the LcSSc, from 59 patients without heart involvement, 10 (16.9%) patients (p = 0.000) showed heart involvement in the follow-up, so heart involvement was significantly lower in the SSc-OS group.
In the SSc-OS group, from 34 patients without PAH at the first visit, none showed new PAH (p = 0.029), and in the LcSSc group, from 62 patients without PAH, 10 (16.1%) patients (p = 0.007) showed new PAH in the follow-up; therefore, new presence of PAH was significantly lower in the SSc-OS group.
In the SSc-OS group, from 26 patients without arthritis at the first visit, 4 (15.4%) patients (p = 0.007), and in the LcSSc group, from 62 patients without arthritis, 2 (3.2%) patients (p = 0.008) showed arthritis, which was significantly higher in the SSc-OS group.
In the SSc-OS group, from 34 patients with normal FTP at the first visit, 1 (2.9%) patient showed mild involvement in the FTP study, and in the LcSSc group, from 59 patients who had normal FTP, 2 (3.4%) patients showed evidence of severe involvement in the FTP study (p = 0.083); the difference was not significant (p = 0.317).
In the SSc-OS group, 16 (45.7%) patients used immunosuppressant drugs (1 [2.9%] AZA, 13 [37.1%] MMF and 2 [5.7%] MMF + cyclosporine), but in the LcSSc group, 37 (56.9%) patients used immunosuppression drugs (3 [4.6%] AZA, 2 [3.1%] CFM, 30 [46.2%] MMF and 2 [3.1%] cyclosporine); there was no significant difference between the two groups (p = 0.273).
In the SSc-OS group of 11 patients with non-specific pattern, 4 (36.4%) patients were converted to scleroderma pattern (3 [75%] patients with SSc-pSS and 1 [25%] with SSc-RA) and in the LcSSc of 5 patients with non-specific pattern, 3 (60%) patients were converted to scleroderma pattern. In the LcSSc group, from 19 patients with early scleroderma pattern, 9 (47.4%) patients were converted to active scleroderma pattern and 2 (10.5%) patients were converted to non-specific pattern, but in the SSc-OS group of 9 patients with early scleroderma pattern, 1 (11.1%) patient was converted to active scleroderma pattern and 2 (22.2%) were converted to non-specific pattern. Also, of 26 patients with active scleroderma pattern, 3 (11.5%) were converted to early scleroderma pattern, and 5 (19.2%) were converted to late scleroderma pattern, but in the SSc-OS group of 9 patients with active scleroderma pattern, 2 (22.2%) were converted to non-specific pattern, and none of them became late pattern.
Therefore, the evidence of change in capillaroscopic patterns was significantly lower in the SSc-OS group, and patients with LcSSc had more progression of their capillaroscopic changes (p = 0.000).
Discussion
In this study, we evaluated the clinical, laboratory, and capillaroscopy manifestations of 70 patients with SSc-OS and their subgroups and compared their organ involvement including musculoskeletal, cardiopulmonary and GI symptoms, DU, and PAH, and their progression in the follow-up with 65 patients with LcSSc.
In our study on SSc-OS patients, the prevalence of patients with SSc-SS (64.3%) was higher than the others, followed by SSc-RA (15.7%), SSc-myositis (12.9%), and SSc-SLE (7.1%). Similar to our study, in the research conducted by Wielosz et al. [1] on 126 patients, the prevalence of overlap of pSS with scleroderma was higher than the others, followed by RA and PM.
In another study conducted by Scherlinger et al. [27] on 534 patients, the prevalence of SSc-RA was higher than the others, followed by pSS and SLE. In a meta-analysis conducted by Elhai et al. [28] on 403 patients with SSc, after autoimmune thyroid disease (10.4%), the most common associated syndrome was pSS (7.7%), followed by DM/PM (5.6%), RA (4.2%), and SLE (2.6%). In this regard, in the study carried out by Foocharoen et al. [29], the prevalence of SSc-PM (70.6%) patients was higher, followed by SSc-SLE (14.7%), SSc-RA (13.2%), and SSc-PM-SLE patients (1.5%).
In our study, 67 (95.7%) patients were female, and there was no significant difference in age between the two groups (OS vs. LcSSc), but in the study conducted by Scherlinger et al. [27], the mean age of the patients with SSc-OS was higher than in our study (62.5 ±14.5 years); like our study, most of the patients were female (26 patients (76%)). In the study conducted by Foocharoen et al. [29] the age of the patients in the OS group (especially SSc-SLE) was younger than the SSc patients. Also, in our study, the mean age of SSc-SLE patients was lower than those with SSc-OS.
In our study, arthritis was significantly higher in the SSc-RA group and evidence of ILD was significantly higher in the SSc-SLE group than the other subgroups, and there was no significant difference between the subgroups in the involvement of other organs.
In our study, DU in patients in the SSc-OS group was significantly lower than the LcSSc group, and new DU was not seen in the SSc-OS patients in the follow-up. Similar to our study, Avouac et al. [30] observed that the association of autoimmune diseases with SSc was with milder symptoms, such as limited skin involvement and fewer DU; also, in the study conducted by Moinzadeh et al. [31], the prevalence of DU in the SSc-OS group was lower than in the SSc patients.
Similar to our study, Wielosz et al. [1] reported that the prevalence of DU was significantly lower in the SSc-OS than the SSc groups, but unlike our study, in the research carried out by Scherlinger et al. [27], the prevalence of DU was similar between overlap SSc and non-overlap SSc patients.
In our study, history of RP was significantly lower in the SSc-OS patients than the LcSSc group, and there was no significant difference in the duration of RP at the onset of disease between groups.
In our study, there was no significant difference between the SSc-OS and the LcSSc patients in pulmonary and cardiac involvement, PAH, and FTP distance. Similar to our study, Scherlinger et al. [27] found no significant difference in PAH, cardiac or pulmonary involvement between the overlap and non-overlap SSc patients; moreover, in the study conducted by Foocharoen et al. [29], there was no significant difference in the prevalence of cardiopulmonary involvement between SSc and SSc-OS patients. In a study carried out by Fairley et al. [2], PAH was more common in SSc patients than OS patients, but this difference was not significant and there was no significant difference in ILD between the groups.
In our study, GI symptoms, including dysphagia, heart burn, difficult swallowing, feeling of being full, and diarrhea were significantly lower in the SSc-OS than the LcSSc patients, but the prevalence of constipation was not significantly different. Unlike our study, Foocharoen et al. [29] found no significant difference in GI symptoms between SSc and SSc-OS patients.
In our study, arthritis was significantly higher in the SSc-OS than LcSSc patients and was observed in the SSc-RA group more than the other subgroups. Similar to our study, in a study by Wielosz et al. [1], the prevalence of arthritis was significantly higher in the SSc-OS than the SSc patients.
In our study, the rate of positivity of ANA, dsDNA, ACA, anti-LA, anti-Sm, anti-Jo1, ACPA, ACLA, CRP, ESR, low C3, and low C4 was not significantly different between the two groups, but that of SCL70 was significantly lower, and the rate of positivity of RF and anti-RO was significantly higher in the SSc-OS patients. Similar to our study, in the research conducted by Wielosz et al. [1] the prevalence of anti-RO was significantly higher, and prevalence of SCL70 was significantly lower in the OS than SSc patients.
Among the subgroups, the rate of positivity of dsDNA and anti-Sm was higher in the SSc-SLE patients; also, the positivity of anti-RO was higher in the SSc-SLE and SSc-pSS patients, and the rate of positivity of RF and ACPA was higher in the SSc-RA patients. However, there were no significant differences in other tests. Like our study, Foocharoen et al. [29] reported that the frequency of ACPA was higher in the SSc-RA patients than other groups.
In our study, the non-specific capillaroscopy pattern was higher, and the scleroderma pattern was lower in the SSc-OS than LcSSc patients, but there was no significant difference between the subgroups, and the evidence of progression of capillaroscopy pattern was significantly lower in the SSc-OS than LcSSc patients. In contrast, in the study conducted by Nagy et al. [10] there was no significant difference between the SSc and SSc-OS patients in capillaroscopy patterns.
In our study, no significant relationship was found between capillaroscopy patterns and organ involvement including lung involvement, DU, PAH, abnormal skin score, and arthritis, but in the study by Markusse et al. [7] on 287 SSc patients, severe nailfold capillaroscopy patterns (active and late scleroderma pattern) were associated with a high risk of cardiopulmonary involvement (ILD and PAH).
In our study, the evidence of progression of lung involvement, PAH, cardiac involvement, skin score, and DU was significantly lower in the SSc-OS than LcSSc patients, and new DU was not seen in the SSc-OS patients; also, the progression of skin score was significantly lower in the SSc-OS than LcSSc patients. Unlike our study, Scherlinger et al. [27] found no significant difference in the development of PAH, cardiac or pulmonary involvement, or DU between the overlap and non-overlap SSc patients. This might be due to the small number of overlap patients compared to non-overlap SSc patients (34 vs. 290).
In our study, the number of patients with SSc-OS who took immunosuppressive drugs was lower than in the patients with LcSSc, but in another study, in the follow-up, patients with OS had a higher need to receive immunosuppressive drugs compared to the SSc patients [29].
Study limitations
Our study had some limitations as follows: first, the number of patients examined in this study was limited. Second, the number of patients referred for follow-up and its duration was small. Third, most of the OS patients were in the SSc-pSS group, so the results when comparing groups should be reevaluated on more patients in future studies. The fourth limitation of our study was not assessing other SSc-related autoantibodies (i.e. anti-RNA polymerase III, anti-Pm/Scl or anti-fibrillarin).
Therefore, the need for studies on a larger number of patients with longer duration and other OS groups is recommended as it seems that the presence of overlap manifestations makes the major organ manifestations less severe with a better follow-up progression.
Conclusions
In SSc-OS patients, the most common subgroup was SSc-pSS. The DU, cardiopulmonary, GI involvement, and PAH in patients with SSc-OS were significantly lower than in the LcSSc patients. Musculoskeletal involvement such as arthritis was more commonly seen in SSc-OS patients, especially in the SSc-RA subgroup. The progression of skin score was significantly lower in the SSc-OS patients; also, non-specific patterns in capillaroscopy were significantly higher than in the LcSSc patients, and the evidence of progression and changes in capillaroscopic patterns was significantly lower in the SSc-OS than the LcSSc group. In follow-up, new DU and the evidence of progression of lung involvement and skin score were significantly lower in the SSc-OS than LcSSc patients.