Introduction
Autoinflammatory diseases (AIDs) cover a spectrum of diseases which lead to chronic or recurrent inflammation caused by activation of the innate immune system, typically in the absence of high titre autoantibodies [1]. The most typical AIDs are hereditary monogenic diseases. The most common monogenic AIDs are familial Mediterranean fever (FMF), NLRP3- associated autoinflammatory diseases, also known as cryopyrin-associated autoinflammatory syndrome (NLRP3-AIDs, CAPS), tumor necrosis factor receptorassociated periodic fever syndrome (TRAPS) and mevalonate kinase deficiency (MKD, formerly known as hyperimmunoglobulin D syndrome, HIDS). NLRP3-AIDs are further divided into 3 clinical forms: Muckle-Wells syndrome (MWS), familial cold autoinflammatory syndrome (FCAS) and chronic infantile neurologic cutaneous and articular syndrome (CINCA) [2].
Definitions of autoinflammatory diseases underline the presence of recurrent autoinflammatory episodes without presence of autoimmune diseases and without the primary pathogenic role of the adaptive immune system [3]. Although this statement seems general, it allows one to exclude fevers without a rise in inflammatory markers. Documented elevated inflammatory markers are an obligatory criterion for suspicion of AIDs. Chronic and recurrent inflammation leads to both acute disease and with time to irreversible damage [4].
Monogenic AIDs are sometimes named autoinflammatory or hereditary recurrent fevers (HRF) [2] and are also known as hereditary periodic fever syndromes (HPFS), defined as three or more episodes of unexplained fever in a six-month period, occurring at least seven days apart [5]. Common opinion, supported by the name “periodic”, suggests that autoinflammatory symptoms need to present with regular, remitting pattern. However, the clinical picture without cyclic recurrences but with constant inflammation and subsequent damage secondary to chronically raised inflammatory markers is seen in adulthood. Adult patients with long diagnostic delay escape HPFS definitions at the moment of diagnosis [6].
Many research papers avoid giving a definition, probably because every attempt of AID generalisation can be detrimental.
Current situation in Poland
AIDs are underdiagnosed worldwide [7–9]. In Poland adult patients are commonly misdiagnosed with other systemic conditions, probably due to low awareness of the disease among Polish physicians. There are only small series and case reports in Polish literature concerning paediatric patients, mainly with periodic fevers with aphthous stomatitis, pharyngitis, and adenitis (PFAPA), polygenic and self-limiting syndrome, and non-monogenic AIDs [10–12].
In our observation the term familial Mediterranean fever (FMF) is best known to rheumatologists and the AID group is colloquially named “FMF-like diseases”. Unfortunately it is misleading as it suggests geographical descent of patients. As from 2017 the national drug programme of interleukin (IL)-1 inhibitor (anakinra) treatment for children and adults with specific autoinflammatory syndromes is available in selected centres of clinical immunology, proper patient diagnosis is a critical issue as it can result in highly effective treatment.
Rheumatologist perspective
Autoinflammatory conditions due to generalised, unexplained symptoms might be referred to rheumatologists. Therefore, to seek AIDs cases we conducted a survey among members of the organization of Young Rheumatologists of the Polish Rheumatology Society. We reached via email over 200 medical doctors working as rheumatologists, including all clinical centres in Poland. We identified only one case of suspected MWS, 2 families with tumor necrosis factor receptor-1 associated periodic syndrome (TRAPS) and 1 FMF case. Unfortunately, due to the lack of feedback about negative cases, we cannot consider this method accurate to conclude on the actual occurrence of AIDs among patients treated by rheumatologists.
Herein we describe 2 AIDs cases to increase awareness of AIDs and to illustrate diagnostic and treatment issues in Poland.
Case 1
The 24-year-old Armenian female was referred to a rheumatologist by a surgery department due to recurrent fevers, symptoms of acute peritonitis, diarrhoea, migrating arthritis, muscle pain and maculopapular rash. Laparoscopy and laparotomy were performed due to acute abdominal pain which did not reveal any pathology, as well as imaging studies (ultrasounds, computed tomography of abdomen). The patient was readmitted to the surgery department 3 times with the same symptoms, which resolved within 3–4 days. Acute gastric pain was the first manifestation starting at the age of 4, accompanied by large joints lasting usually 3–4 days from the age of 10. About the age of 15 episodes were exacerbated with concomitant fever (38–39°C), arthralgia, myalgia, diarrhoea, nausea, vomiting and skin eruptions (Table I).
Table I
Case presentations
Symptoms were preceded by flu-like symptoms, resolved spontaneously and recurred a few times in a month. The family history revealed similar symptoms in her mother and brother, who was diagnosed with MEFV gene positive FMF (M694V homozygote). The patient’s basic laboratory tests revealed only increased CRP and ESR (Table I). Antinuclear antibodies (ANA) were present (1 : 640), but without autoantibodies specific for connective tissue diseases. Non-steroid anti-inflammatory drugs (NSAIDs) were initially administered with rapid relief of symptoms and decrease in acute phase parameters. Based on clinical data a diagnosis of FMF was posed. The patient fulfilled the clinical criteria for FMF diagnosis (Table II) [13].
Table II
* The requirements for diagnosis of FMF are ≥ 1 major criteria, or ≥ 2 minor criteria, or ≥ 1 minor criterion plus ≥ 5 supportive criteria, or ≥ 1 minor criterion plus ≥ 4 of the 5 supportive criteria. Typical attacks are defined as recurrent (≥ 3 of the same type), febrile (rectal temperature of 38°C or higher, and short (lasting between 12 hours and 3 days). Incomplete attacks are defined as painful and recurrent attacks that differ from typical attacks in one or two features, as follows: 1) the temperature is normal or lower than 38°C; 2) the attacks are longer or shorter than specified (but not shorter than 6 hours or longer than a week); 3) no signs of peritonitis are recorded during the abdominal attacks; 4) the abdominal attacks are localized; 5) the arthritis is in joints other than those specified. Attacks are not counted if they do not fit the definition of either typical or incomplete attacks.
Long-term treatment with colchicine (1 mg/day) was started, with good control of symptoms. No manifestations of amyloidosis or other complications were present at the time of diagnosis but she was soon lost to follow-up, continuing her treatment in Armenia.
Case 2
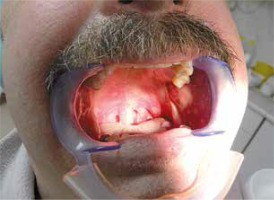
The 54-year-old man was referred to a rheumatologist due to pyrexia of unknown origin (Table I). Family history revealed only 3 cases of unexplained deaths in infancy in the patient’s uncles and aunts. The patient complained of “a flu that won’t go away” with generalised muscle and joint pain, lasting for 4 years. During that period he underwent open liver biopsy and splenectomy that confirmed only focal nodular hyperplasia and simple cyst respectively. Long courses of multiple antibiotics were unsuccessful. Diffuse muscle tenderness was a constant finding but only arthralgia without joint inflammation was noted. Episodic conjunctivitis was present and only one episode of mouth ulceration occurred during the whole follow-up (Fig. 1).
Sensineuronal hypoacusis was documented. Polymyalgia rheumatica with subclinical giant cell arteritis was suspected. Although temporal arteritis classification criteria [14] were met (age > 50, raised ESR, temporal headache) 5 cm long temporal artery biopsy and large arteries visualisation revealed no vasculitis. Differential diagnosis also encompassed atypical Cogan syndrome. However, no typical eye inflammation was observed and his brain MRI revealed only cortical atrophy. Extensive diagnostics revealed no underlying cause of raised inflammatory markers.
Due to a suspected autoimmune condition treatment with cyclophosphamide, methotrexate, azathioprine, cyclosporine and plasmapheresis was introduced without effect. Retrospective analysis of his medical history revealed that he had constant elevation of inflammatory markers documented in his records from the age of 20. Therefore, AID was suspected. However, no mutations in Sanger analysis in MEFV, NLRP3 and TRAPS genes were found.
At that time next generation sequencing was not available for us. Unclassified autoinflammatory syndrome, most likely MWS, was posed as a diagnosis of exclusion. Due to unavailable biologic treatment the patient continued on GCs (requiring a minimal dose of 10 mg prednisone to control his symptoms), NSAIDs (maximal daily doses improved his muscle pain) and colchicine (maximal tolerated dose 2 mg/day) with resolution of fevers and partial control of his symptoms but without normalisation of inflammatory markers.
At the age of 64 the patient developed proteinuria. Amyloidosis was confirmed on sigmoid biopsy. With diagnosis of AA amyloidosis secondary to AIDs he was qualified for a Polish national programme of anakinra treatment shortly after establishing it. Unfortunately, he died shortly after induction of this treatment because of confirmed myocardial infarction. He left his doctors wondering about the chances of reducing his cardiovascular risk in case of earlier treatment availability.
Discussion
Why is diagnosis a problem?
The short AIDs and HPFS definitions may be a source of misunderstandings, because it is a highly heterogeneous group. “Hereditary” implies familiar distribution. However, family history might be a clinical hint as in case 1, while on the other hand a significant number of AD mutations are spontaneous. Duration, frequency and severity of attacks may vary within a specific syndrome. Irregularity is a feature of TRAPS. In CAPS attacks can be very frequent or constant. In FMF and MKD they can be either irregular or regular.
The most regular periods between fevers are observed in PFAPA – a self-limiting, polygenic childhood disease, occasionally found in young adults. The PFAPA is an example of disease that in the era of vast diagnostics can only be diagnosed based on clinical observation: concluding from regularity of symptoms resembling adenitis, that resolves in spite of lack of antibiotic administration.
Chronic fever of unknown origin (FUO) may be ignored by a practitioner after exclusion of infections and malignancy with an excuse that persisting inflammation is clinically irrelevant. However, complications of chronic inflammation become apparent with time: case 2 presented without symptomatic amyloidosis at the initial work up but developed it during follow-up [15, 16].
Epidemiology
The AIDs symptoms more often start in early childhood, but both late onset and survival into adulthood can be expected. Monogenic AIDs occur as commonly in males as in females (autosomal mutations). Current prevalence in Poland is not known. The highest FMF carrier frequency has been estimated to 1 : 5 in Turks, resulting in prevalence of 1 : 1000 (lower than expected from simple Mendelian calculation), 1 : 7 in Armenians (but resulting in 1 : 500 prevalence), 1 : 135 in Ashkenazi Jews who were inhabiting Central Europe, in contrast with up to 1 : 5 in non-Ashkenazi Jews [17–19].
Prevalence of NLRP3-AIDs in France was calculated at maximum 1 : 360,000 [20]. Prevalence of TRAPS is approximately 1 per million [21]. There are only about 300 MKD case descriptions in the literature, coming mainly from France and Denmark. Both countries significantly contribute to research on AIDs. Analogically, most FCAS descriptions come from the USA. Therefore, the actual number of cases in countries with lower awareness of monogenic AIDs is likely higher than reported [22].
Aetiology and pathogenesis
In contrast to autoimmune diseases, aetiology of monogenic AIDs is well characterised by autosomal mutations in single genes. There is no antigen-dependent activation of immune reaction typical for acquired immunity (manifested by presence of autoantibodies or autoantigen-specific T cells) and lack of complicated MHC associations. “Autoinflammation” attributes to increased innate immunity, autoimmunity to dysregulated adaptive immunity (e.g. in systemic lupus erythematosus).
The AIDs pathogenesis may seem to be more direct and simple compared to acquired immunity. Innate immunity activations dependent on molecular pattern, based on 3 types of pattern recognition receptors (PRR). They encompass endocytic receptors, e.g. mannose receptors, secreted PRRs, e.g. C-reactive protein and signalling PRRs, e.g. Toll-like receptors or NOD-like receptors. The latter form an inflammasome, which is a multiprotein oligomer responsible for the activation of inflammatory responses through IL-1 activation. AIDs are generally considered signalling defects. Most of them result from inflammasome function stimulation. It can be direct, caused by gain of function mutation as in CAPS – the disease currently considered the prototype of IL-1 driven autoinflammation. It can also be indirect, due to lack of inflammasome inhibition by pyrin, probably like in FMF. Other rare autoinflammatory diseases may result from multiple, still poorly understood, dysregulated pathways, e.g. interferon mediated. The pattern of tissue injury in AIDs is also principally different from that observed in autoimmune diseases [23, 24].
Diagnosis
The possibility of AIDs should be raised if episodes of fever with raised inflammatory markers persist for months, but the patient’s general state remains good enough to exclude malignancies and infections. As clinical manifestations are fairy pathognomonic, the diagnosis frequently comes after follow-up, as in the presented cases.
Common AIDs symptoms are unspecific: fever, rash, arthralgia. Other manifestations include serositis, arthritis, meningitis, uveitis, lymphadenopathy and splenomegaly. Abrupt episodes of fever accompanied by other manifestations are suggestive but are not mandatory for the diagnosis. The duration and periodicity of fevers can be useful discriminators of AIDs (Table III).
Table III
Types and typical presentations of monogenic autoinflammatory diseases
| Type of AID | Typical age at first manifestation | Mean time from symptoms to diagnosis (years) | Frequency of seizure | Regularity of seizures | Duration of a single seizure (days) | Fever (°C)* | Main manifestations at seizure | Manifestations at periods between seizures | Complications | Mean CRP during attack (mg/l) | Mean CRP between attack (mg/l) | Inheritance | Genetic tests | Treatment | Response to treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FMF | < 20 | 1.8 [7, 8, 15] | Every 4–8 weeks, can be induced** | Possible | 1–3 | 38–41 with chills | Serositis, rash, monoarthritis, atypical (in Polish patients) | Signs absent; rarely chronic rise of CRP, neutrophilia, thrombocytosis, anaemia | Amyloidosis | 115 [20] | 4 | AR, rarely AD pattern | MEF | Chronically colchicine, biologics | Usually satisfactory |
| MWS | Variable | 20.6 [15, 24, 25] | Variable | Irregular | 1–3 or constant | Sometimes with chills, more frequent in children | Rash, conjunctivitis, arthralgia, strong pain of extremities, periodic weakness | Signs can be present, chronic rise of CRP, neutrophilia, thrombocytosis, anaemia | Amyloidosis, sensineuronal hearing loss | About 20 – regardless of exacerbation, (elevated in about 90% of patients) [26], occasionally reported > 160 [27] | AD | NLRP3/CIAS1, somatic mutations possible | Biologics | Variable | |
| TRAPS | < 20 | 5.8 [7], 6.5 [15] | Variable (days-months intervals) | Irregular | 15 in severe disease, 5–9 in mild | > 38, max. 41, can be absent in adults [25] | Arthralgia, localised muscle pain, migratory rash, conjunctivitis, eye lids oedema, stomach ache, vomiting, diarrhoea | Signs absent; rarely chronic rise of CRP, neutrophilia, thrombocytosis, anaemia | Amyloidosis | 95*** [25] | 25*** | AD incomplete penetrance | TNFRSF1A | Cortico-steroids, biologics | Variable |
MF – familial Mediterranean fever, MWS – Muckle-Wells syndrome, TRAPS – tumor necrosis factor receptor-1 associated periodic syndrome. Data are presented analogically to Table I.
The duration of fever was proved to be helpful in distinguishing between FMF (shortest – mean 1–3 days) and TRAPS (longest – up to 15 days) [28]. However, in adults periodicity may only manifest by fluctuations of CRP levels [29]. Inflammatory markers become elevated during seizures, but can also remain abnormal in between. Serial, regular measurements of body temperature and CRP can be helpful if attacks are not distinctive or hard to observe. Clinical characteristics were quite different in both of our presented cases (Table I).
The longer, more remittent and intensive the inflammatory periods, the higher the probability of amyloidosis: e.g. amyloidosis is not typically found in PFAPA. Most cases of AIDs begin in childhood. Clinical presentation in adults might be milder or atypical forms with less pronounced periodicity making diagnosis challenging. Moreover, knowledge about AIDs comes mostly from observations in children, which leads to including AIDs in differential algorithm more often in the paediatric population. However, in our own experience diagnosis of an adult patient with AA amyloidosis of unknown aetiology resulted in prompt diagnosis of AID in his children [6, 8].
A key point in differential diagnosis is to realise that the patient’s pyrexia is relevant although nothing else but an autoinflammatory process. It is usually subject to exclusions. Sometimes malignancies and chronic infections are first excluded by the simple fact of a long disease course without fatal progression. In daily routine, rheumatology is considered a speciality focused on anti-nuclear antibodies (ANA). This test is sometimes overused as a screening laboratory procedure [30]. Presence of autoantibodies is only a part of many immune processes. Although AIDs are a form of innate immunity dysregulation, that may trigger nonspecific adaptive immunity activation and autoimmunisation. Polyclonal hypergammaglobulinemia is frequently found in AIDs [29]. Presence of autoantibodies, especially low-titre and nonspecific, does not exclude AIDs [31]. High-titre and specific autoantibodies are usually absent in AIDs without accompanying autoimmune diseases.
There have been classification criteria for AIDs proposed and validated in 2015 and 2019 by PRINTO [2, 32]. Although these criteria may at times be helpful in clinical practice, they are explicitly not meant to be employed as diagnostic criteria. In our practice we use them to qualify adult patients for genetic testing.
Ex juvantibus empiric therapy may be helpful for diagnosis. Colchicine is advocated in FMF suspicion. In TRAPS and CAPS, a rapid and spectacular response to IL-1 blockade is observed.
Further characteristics of the most common monogenic AIDs are presented in Table IV.
Table IV
Characteristics of selected monogenic autoinflammatory diseases
| Disease | Characteristics |
|---|---|
| FMF | Is caused by gain-of-function mutations in MEFV gene, encoding the protein pyrin, which induces cryopyrin-related inflammation. Its inheritance was considered AR. However, few patients have only mutation of one allele, suggesting additionally AD inheritance or haploinsufficiency. Ethnic groups with high prevalence are Turks, North Africans, Greeks and Italians. A founder effect is considered a plausible explanation. Recently a new tempting hypothesis was proposed explaining the selection of FMF patients. Yersinia pestis, which was responsible for plague epidemics in the Mediterranean, blocks the inflammasome. FMF provides constant activation of the inflammasome, potentially making patients more resistant against plague. Yersinia outer proteins might potentially become a drug against FMF [33]. Heterozygous carriers of the FMF gene usually do not display clinical manifestations, but they may demonstrate more robust inflammatory responses (high fevers, more dramatic sepsis or extraintestinal Crohn’s disease) or higher incidence of inflammatory diseases e.g. rheumatoid arthritis (RA) [34, 35]. Pattern of joint involvement in FMF differs from RA, encompassing monoarthritis of knee, ankle or wrist, and is non-destructive (with some exceptions reported ). FMF may overlap with Schönlein-Henoch purpura and periarteritis nodosa. Recurrent courses of these diseases may profit from screening for FMF. Typically, attacks of fever are accompanied by pain of the abdomen (most common), chest and joints caused by peritonitis, pleurisy and synovitis respectively. Lower limb skin changes may be mistaken for erysipelas, especially as they are painful. Skin lesions localise under the knees and over the hands. Although some AID features may seem overlapping, FMF does not present with conjunctivitis or periorbital oedema [36]. CRP serum concentration is normal in 50% of FMF patients between attacks, and attacks might be hard to define [37]. SAA serum concentration has higher sensitivity. There is no FMF attack without a rise in CRP serum concentration. Leucocytosis is found in the peripheral blood during attacks. Joint fluid examination reveals neutrophils [13, 38]. Polyclonal gammopathy can be present mainly within IgA – in 80% of patients – but also immunoglobulin D (IgD). Amyloidosis and infertility can be complications |
| CAPS | Cryopyrin gene mutation causes gain of inflammasome activation resulting in 3 different syndromes, classified as CAPS. Cryopyrin is currently termed NLRP3 (nucleotide-binding domain and leucine-rich repeat containing family, pyrin domain-containing 3) – a scaffold protein. Patients with CAPS can be classified under one of three clinical syndromes. Muckle-Wells syndrome (MWS) is the most common, but can overlap with the others. Age of onset of MWS is very different [39]. It manifests with attacks of fevers – sometimes with chills, urticarial rashes, conjunctivitis and fatigue. It progresses to sensorineural hearing loss (due to chronic inflammation in inner ear or cryopyrin dependent cartilage changes) and amyloidosis. Due to multiple manifestations MWS patients are initially referred to different specialists. Rash in CAPS is not itching but burning – in contrast with allergy. Skin biopsy reveals neutrophilic urticaria (considered typical, but sometimes observed in FMF), sometimes with vasculitis. Constant CRP elevation is not uncommon. MWS in children is highly inflammatory with more fevers (50% vs 30%) and abdominal pain, while in adults organ damage is more pronounced with hearing loss and fatigue (see case 2) [40]. Constant meningitis from childhood can result in mental retardation [41]. Chronic infantile neurologic cutaneous and articular syndrome (CINCA) is the most severe form of CAPS, beginning in infancy. In the USA it is descriptively named neonatal-onset multisystem inflammatory disorder (NOMID). Prevalence is estimated to be only several hundred patients in the world, influenced by high mortality [42]. Familial cold autoinflammatory syndrome (FCAS) is the mildest form, of unknown prevalence, manifested by up to one day long episodes of fever and urticaria characteristically triggered by exposure to cold [39] |
| TRAPS | TRAPS results from TNFR1 gene mutation, AD inherited with incomplete penetrance. Most reported patients were of northern European descent but different ethnicities can be affected. First attack after 20 years of age makes TRAPS diagnosis unlikely, but the diagnostic delay is long. Out of the common triad of AIDs symptoms (fever, rash, arthralgia), patchy rash can be quite specific, spreading distally down an extremity. Only the face is spared by the rash. The clinical picture may vary significantly among patients. Attacks can manifest predominantly as serosal (peritoneal, pleural, pericardial), and/or synovial inflammation. Migratory myalgias are common, and are suspected to be caused by fasciitis. Myalgia in FMF is milder and lasts shorter. One muscle group is typically attacked “at the root of a limb”, accompanied by local erythema and pitting oedema. Patients describe it as deep, cramping muscle pain. Abdominal pain is a common, nearly consistent feature, and often severe. Arthritis is rare and limited to a single, large joint. Uveitis, conjunctivitis and periorbital oedema can coexist. There is leucocytosis, anaemia of chronic disease type, moderately lowered complement and polyclonal gammopathy – especially IgA type. Immunoglobulin D (IgD) can also be elevated but usually < 100 IU/ml. Amyloidosis develops in 25–40% – less frequent compared to FMF and CAPS. Steroids are commonly applied. They are effective in prednisone doses > 20 mg/day, so side effects are significant [25, 36] |
| MKD | Is caused by reduced mevalonate kinase (MVK) activity resulting from AR MVK gene mutation. Typical age at first manifestation is below 1 year, and it rarely manifests over 10 years of age. MKD flares lasting 3–7 days occur in irregular intervals precipitated by infection or stress. In addition to fever, rash, arthralgia or arthritis there is cervical lymphadenopathy, occasionally splenomegaly and hepatomegaly, abdominal pain, vomiting or diarrhoea, and aphthous mouth ulceration. Splenomegaly, although uncommon, was proved to be an important discriminatory sign between other AIDs. Total MVK deficiency is rare and results in mevalonic aciduria (dysmorphology, fevers and failure to thrive). Most of its cases are reported in children in Arabic countries. Genetics is the mainstay of MKD diagnosis rather than IgD level, which is not necessarily elevated. However, presence of evidently high IgD concentration (> 100 IU/ml, equals 140 mg/l) offers a diagnostic biochemical tool for the diagnosis. In contrast with MKD, other AIDs lack biomarkers. IgD concentration do not correlate with disease activity. Amyloidosis risk is low [2, 7] |
Genetic testing
Patient ethnicity should be analysed. Mediterranean ancestry makes FMF diagnosis more likely, but other AIDs seem similarly distributed in different populations [43]. There are some distinct ethnic groups in Poland, e.g. Greek emigrants or past and recent Armenian diasporas that are both risk groups for FMF. Increased consanguinity rates in these groups should also be considered. De novo mutations may happen in every ethnic group.
Exact diagnosis is important not only for the implications for therapy but also for genetic counselling [44]. If family history is positive the type of inheritance should be analysed (AR or AD). However, high consanguinity rates may account for the occurrence of AR diseases in two or more successive generations (pseudodominant inheritance, pattern of vertical transmission). Negative familiar history is not an exclusion as a high proportion of patients have de novo mutations.
Nowadays specific genetic tests are more widely accessible. Some of them enable only testing of selected types of mutations – the most common ones. Unfortunately, many AIDs arise from new, somatic mutations. To detect them sequencing the whole gene might be needed as well as evaluation for mosaicism. However, even the best genetic testing is frequently without a conclusion. It cannot be a screening test and always has to be interpreted in the context of clinical data. Low-penetrant mutations in autoinflammatory genes do not occur more frequently in patients with AIDs compared with the general population. Diagnosis can be made by thorough clinical evaluation followed by targeted genetic analysis [45]. Lack of genetic confirmation should not stop ex juvantibus IL-1 inhibitor therapy.
Differential diagnosis in adults
The differential diagnosis of pyrexia of unknown origin is vast. An extensive comparison chart of autoinflammatory diseases is found online at the Autoinflammatory Alliance foundation [46]. Many common inflammatory diseases, such as gout (typically recurrent, which resembles AIDs), activate autoinflammatory mechanisms [47].
Also some autoimmune diseases include autoinflammatory components as in the case of giant cell arteritis accompanied by polymyalgia rheumatica [48]. Adult-onset Still’s disease is sometimes classified as an autoinflammatory disease, but without monogenic inheritance. Some manifestations (high-spiking fevers, arthritis, salmon-coloured rash, serositis, and lymphadenopathy) may resemble AIDs, especially if recurrent. Schnitzler syndrome is another non-monogenic autoinflammatory condition, presenting with urticaria and monoclonal gammopathy.
Treatment
Conventional disease-modifying antirheumatic drug (DMARD) and GCS treatment is only partially effective or completely ineffective. There is no autoaggression against different organs, but substantial damage is secondary to long-lasting inflammation: amyloidosis, cachexia and cardiovascular disease. The pyrogen-related inflammatory response lacks responsiveness to immunosuppression or classical DMARDs as initially tried in case 2 [23, 24].
Cases with only mild or occasional attacks may not require constant treatment. The aim of long-term treatment, especially in severe cases, is to prevent AA amyloidosis (which is an important prognostic factor) and premature cardiovascular disease [49]. Even in the case of achieving good control of symptoms there is still a risk of progression of amyloidosis due to chronic, subclinical inflammation. Serum amyloid A (SAA) seems to be a critical parameter to control [50]. Some untreated cases of AIDs never normalise SAA and their CRP concentrations. The auto-inflammatory diseases activity index (AIDAI) may help to decide on intensity of treatment [51].
In FMF continuous colchicine therapy is highly effective in preventing attacks and systemic amyloidosis, although with 10% resistance. Acute symptoms of FMF such as fever, myalgia, or abdominal pain respond to corticosteroids or NSAIDs but not to colchicine. Colchicine is not a symptomatic treatment, but medication which modifies disease progression. In other AIDs there is no well confirmed response to colchicine. TRAPS can respond to corticosteroids > 20 mg/day or NSAIDS [36, 52].
Anti-cytokine biologic drugs have been used to treat different AIDs accordingly to the crucial role IL-1 in its pathogenesis [53]. The first applied product was anakinra, another is rilonacept, unavailable in Europe, and canakinumab. All preparations effectively relieve clinical symptoms, which is accompanied by the normalization of laboratory inflammatory markers, and prevent the development of amyloidosis [54, 55]. In FMF patients with AA amyloidosis, anti-IL-1 treatment can reverse proteinuria. A further option, both in children and in adults, is canakinumab – another anti-IL-1 blocker [26, 56, 57] – and rilonacept, known as IL-1 Trap (not available in Europe). Anti-tumor necrosis factor α (TNF-α) therapies are generally not effective but interestingly etanercept may ameliorate TRAPS manifestations [58]. Anti-IL-6 receptor therapy with tocilizumab was successfully tried in small series of patients. However, high doses are needed [59].
Assessing cytokine profile might be helpful in choosing the type of biologic drug if it is possible to choose [60].
In the Polish therapeutic programme the IL-1 inhibitor – anakinra is available for treatment of children and adults with AIDs who meet treatment eligibility criteria. Current criteria include CAPS, TRAPS, FMF cases resistant for colchicine, Schnitzler syndrome and cases with AA amyloidosis due to autoinflammation mediated by IL-1. A particular patient’s eligibility for the programme has to be confirmed by the Coordinating Team established by the President of the National Health Fund [61].
Conclusions
In conclusion, the Polish national programme of anti-IL-1 treatment opens new possibilities for the treatment of AIDs. However, they remain underdiagnosed and more awareness is needed [62].