Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation symmetrically affecting joints and leading to progressive cartilage destruction and bone erosion in the late stages of the disease [1]. Pain, swelling, and stiffness of the affected joints, often accompanied by morning stiffness lasting over one hour, usually affect such patients [2]. Nonspecific symptoms such as fatigue, weakness, and loss of appetite also may be observed. The disease commonly progresses slowly and insidiously. Rheumatoid arthritis is a chronic disease characterized by periods of flare-ups and remission, affecting patients both physiologically and psychologically. If left untreated, it causes irreversible joint destruction together with a loss of functionality [3]. Rheumatoid arthritis represents a disease characterized by the infiltration of B lymphocytes, T lymphocytes, and monocytes into the synovial membrane of the joints [4]. This process is preceded by the activation of endothelial cells, and the resulting neovascularization is a distinctive feature of RA synovitis. In the pathogenesis of RA, cytokines play a key role in the immune process [5]. These immune processes are shown to trigger autoimmunity and lead to chronic inflammation and tissue damage. Pain, swelling, and joint damage are caused by pro-inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin-6 (IL-6). These inflammatory cytokines exert their effects by inducing other molecules such as receptor activator of nuclear factor kB ligand, prostaglandins, and matrix metalloproteinases. Receptor activator for nuclear factor κB ligand, by being stimulated by TNF and IL-6, itself stimulates osteoclasts in the synovial membrane, leading to bony destruction [5]. Evaluating disease activity and treatment response is crucial for the management and follow-up of patients. Various scales have been developed to assess disease activity. Disease activity is usually monitored using the widely employed disease activity score, which is known as Disease Activity Score 28 (DAS28). It is calculated by summing the number of swollen joints and the number of tender joints, the visual analog scale of general health, and the ratio of C-reactive protein (CRP) or erythrocyte sedimentation rate (ESR). A DAS28 score above 5.1 reflects high disease activity requiring immediate medical attention or adjustment of treatment. A score in the range of 3.2 to 5.1 indicates moderate activity, a state where continued monitoring and intervention are required when appropriate. Scores from 2.6 to 3.2 represent low activity, which reflects good control, yet vigilance is required. Scores below 2.6 indicate remission, where the disease is inactive, the symptoms are minimal, and health status may be considered stable [6]. Inflammatory biomarkers such as ESR and CRP are commonly used to monitor disease activity in rheumatic diseases, though their sensitivity and specificity are low [7]. Therefore, there is a need for new parameters that better reflect disease activity [8]. Recently, components of complete blood cells have been used to evaluate systemic inflammation in both rheumatic and non-rheumatic diseases. Examples include the systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), and pan-immune-inflammation value (PIV). These metrics, derived from hematological evaluations, measure the intensity of inflammation and have been validated as prognostic indicators in various malignancies, though primarily inferred from oncological outcomes [9]. In esophageal cancer, there is a strong association between clinical outcomes and tumor-infiltrating lymphocytes with the SII [10]. Long-term outcomes in patients suffering from colorectal cancer have been speculated to be effectively predicted using the PIV [11]. Neutrophil, platelet, and lymphocyte levels are components of the formula used to determine the SII. It is not clear what its role is in prognosis evaluations in cancer, yet its importance is noted since it increases for certain cancers. It is hard to define the role of the SII, such as its advantages for non-small cell lung cancer, pancreatic tumors, and gastric carcinoma, often used as examples to illustrate this index. As an inflammatory marker, it is elevated when inflammation is continuous and an individual has a tumor [12, 13]. The SII index is derived from the product of neutrophils and platelets, divided by the number of lymphocytes. An elevated SII value infers an increase in neutrophils and platelets, but a decline in the lymphocyte count [14]. This perhaps implies a considerable inflammatory response. Newly recognized, the SIRI biomarker is derived from the neutrophil, monocyte, and lymphocyte ratio. The SIRI’s role as an important marker has been established in the emergence and progression of several malignant cancers, according to past studies [15, 16]. Erre et al. [17] recently stated that SIRI can reflect chronic inflammation in rheumatic conditions more broadly, rather than being limited to RA alone, as seen in their investigation.
This study aimed to investigate the relationship between PIV, SII and SIRI and disease activity in RA, characterized by chronic inflammation and immune system involvement, and to provide new insights into the clinical implications of RA.
Material and methods
A retrospective investigation was undertaken on 97 individuals diagnosed with RA, according to the American College of Rheumatology (ACR) – European Alliance of Associations for Rheumatology (EULAR) criteria, compared with 51 seemingly healthy individuals in this case-control inquiry. Acute infections, diabetes mellitus, malignancies, fresh glucocorticosteroids (GCs) administration, along with granulocyte-colony stimulating factor (G-CSF), could potentially modulate levels of lymphocytes and neutrophils – these were the exclusion criteria from the patient group. No inflammatory sicknesses, quick or prolonged infections, malignancy in motion, diabetes, or substances that possibly affect neutrophil and lymphocyte concentrations were the exclusion criteria for the control group. Routine hematological and biochemical analyses were carried out for both cohorts, inclusive of RA patients. Assessing disease activity was succinctly done via DAS28 on RA patients. A juxtaposition of immuno-inflammatory traits from both groups, and RA patients based on disease activity levels, was executed as well. This investigation included subjects who visited the Rehabilitation and Physical Therapy Division in the period from January 1st to December 31st, in the year 2023, at the Medical Faculty of Samsun University. Following physical assessments of all participants across both varieties, demographic and clinical characteristics were noted. Concerning full blood cell parameters, neutrophils (109/l), lymphocytes (109/l), monocytes (109/l), platelets (109/l), ESR (mm/h), and CRP (mg/l) were recorded. The SII was calculated from the neutrophil count multiplied by the platelet count and divided by the lymphocyte count. Systemic inflammation response index is defined as follows: SIRI = N × M/L, where N, M and L are neutrophil, monocyte and lymphocyte counts, respectively. The PIV is calculated as the product of the neutrophil count, platelet count, and monocyte count, divided by the lymphocyte count [20]. For RA patients, disease activity was determined using the DAS28 score. This score carries significant weight in daily practice and has been endorsed by EULAR and ACR. A high disease activity is indicated by a DAS28 score above 5.1. Moderate activity lies between scores of 3.2 and 5.1, while low activity falls between 2.6 and 3.2. The threshold of remission is a DAS28 score below 2.6. The DAS28 score is calculated using the formula: 0.56*sqrt (tender 28) + 0.28*sqrt(swollen 28) + 0.70*Ln(ESR) + + 0.014*global health condition. The sample size was determined using the G*Power 3.1.9.7 power analysis program. As a result of the analysis, it was determined that the minimum required sample size was 90 (Test family: t-test, Statistical test: Means: difference between two independent means (two groups), power analysis type: a priori: compute required sample size – given α, power and effect size).
Results
In this study, 97 patients diagnosed with RA and 51 healthy individuals were included. Of these patients, 25 (25.77%) were male, and 72 (74.23%) were female. Among the healthy individuals, 20 (39.22%) were male, and 31 (60.78%) were female. There was no significant difference in gender ratios between the patient and control groups. The average age of the patients was 54.77 ±10.73, while the average age of the control group was 46.92 ±14.04. The average age of the patients was significantly higher than that of the healthy individuals (p = 0.002). The laboratory findings of the participants are presented in Table I.
Table I
Laboratory findings of patients and healthy individuals
The values of white blood cells (WBC), neutrophils, monocytes, platelets, ESR, CRP, SII, PIV, and SIRI in the patients were significantly elevated compared to the control group (Table I). Conversely, the lymphocyte counts in the patients were markedly lower than those of the control group (see Table I). The average DAS score recorded for the patients was 3.69 ±1.38. A correlation analysis was conducted to explore the association between DAS scores and the parameters SII, PIV, and SIRI. The results indicated a positive correlation between DAS scores and SII (correlation coefficient: 0.316, p = 0.002), PIV (correlation coefficient: 0.343, p < 0.001), and SIRI (correlation coefficient: 0.361, p < 0.001).
Out of 97 patients, 64 (65.98%) were experiencing an active phase of RA, while 33 (34.02%) were in remission. Within the group of active patients, 19 (29.69%) were male and 45 (70.31%) were female. For those in remission, there were 6 males (18.18%) and 27 females (81.82%). The average age for patients in the active phase was 54.23 ±11.70 years, compared to an average age of 55.82 ±8.62 years for those in remission. No significant differences in sex distribution or average ages were observed between the 2 groups. Additionally, the average DAS was 4.50 ±0.94 for patients with active disease, while it was markedly lower at 2.12 ±0.36 for patients in remission, with a p-value of less than 0.001, confirming statistical significance. Laboratory results for both patient groups are detailed in Table II.
Table II
Laboratory findings of patients in the active and remission phases
The levels of WBC, neutrophils, ESR, CRP, SII, PIV, and SIRI in patients during the active phase were significantly elevated compared to those in remission (Table II). A binary logistic regression analysis was conducted to assess how well the SII, PIV, and SIRI metrics could predict the duration of RA. The findings from this analysis are shown in Table III.
Table III
Binary logistic regression analysis results of SII, PIV, and SIRI parameters for the RA disease period
| Parameter | Predicted rate (%) | Odds ratio (95% CI) | p |
|---|---|---|---|
| SII | 75.26 | 1.004 (1.001–1.007) | 0.003 |
| PIV | 71.13 | 1.003 (1.001–1.005) | 0.002 |
| SIRI | 72.16 | 4.357 (1.785–10.639) | 0.001 |
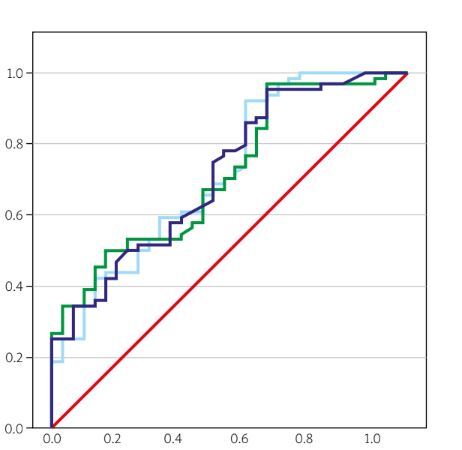
In the evaluation of RA disease activity, the predictive accuracy of SII, PIV, and SIRI parameters was assessed using receiver operating characteristic (ROC) analysis. The results of this analysis are presented in Table IV.
Table IV
ROC analysis results of SII, PIV and SIRI parameters for RA disease stage
| Parameter | AUC (95% CI) | Cutoff | p | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|
| SII | 0.717 (0.612–0.821) | 611.45 | < 0.001 | 57.81 | 60.61 |
| PIV | 0.719 (0.610–0.827) | 323.88 | < 0.001 | 60.94 | 63.64 |
| SIRI | 0.717 (0.611–0.823) | 1.18 | < 0.001 | 59.38 | 63.64 |
The SII measurements above 611.45, with a sensitivity of 57.81% and specificity of 60.61%, are considered a risk factor for active RA. Similarly, PIV values over 323.88, exhibiting 60.94% sensitivity and 63.64% specificity, along with SIRI values above 1.18, showing 59.38% sensitivity and 63.64% specificity, are also recognized as risk factors for active RA (Table IV and Fig. 1).
Fig. 1
The ROC curve of SII, PIV, and SIRI parameters for the RA disease period.
PIV – pan-immune-inflammation value, SII – systemic immune-inflammation index, SIRI – systemic inflammation response index.

The DAS scores, when broken into subgroups of patients active in disease conditions, are as follows: a score between 2.6 and 3.2, level 1, indicates a diagnosis of low disease activity. Moderate disease activity receives a diagnosis with a score from 3.2 to 5.1 – i.e. level 2. At level 3, a score of 5.1 or higher indicates high disease activity. The details of patients with high, moderate, and low disease activity as they relate to the results of SII, PIV, and SIRI are summarized in Table V.
Table V
Results of SII, PIV, and SIRI parameters for subgroups based on DAS scores
The SII, PIV, and SIRI scores in the DAS 3 subgroup were found to be higher than those in the DAS 2 subgroup (Table V).
Discussion
The study explored the relation of PIV and SII with active RA. Compared to 51 controls in a healthy state, 97 RA patients had elevated values of the parameters WBC, neutrophil, monocyte, and platelets, as well as SII, PIV, and SIRI – all significantly higher. Observing the connection between RA activity and those markers, they were notably elevated in patients with full-blown disease: PIV, SII, and SIRI were indeed high. Analysis of ROC confirms that above certain limits, PIV, SII, and SIRI values could signify active RA; the precise numbers being PIV – 323.88, SII – 611.45, and SIRI – 1.18. For non-invasive procedures, CRP and ESR are endorsed for use as markers. Disease activity assessment in RA patients is where they belong, even today. They have qualities such as low costs, high availability, and straightforwardness in measurement. But one should remember that high serum CRP levels could be affected by other types of inflammation too. Erythrocyte sedimentation ratio is slow and insensitive in response to shifts in disease activity when compared to CRP; thus, it is a rare choice. Novel markers are a necessity; they require quickness and frugality, as well as availability, for evaluating disease activeness. Persistent inflammation, an unwelcome feature in RA, negatively affects the performance of neutrophils, lymphocytes, and platelets. The immune system, a two-part composition, includes B and T lymphocytes. Thorough functionality is seen in B lymphocytes; while T lymphocytes, in RA, display subdued actions. This brings about immune malfunction. Conversely, the overstimulated T lymphocytes reach their limit [18, 19]. Some of these changes have recently been detected using more complex complete blood count-derived systemic inflammatory markers, such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR). Among those inflammatory markers are SII and PIV. Systemic immune-inflammation index includes neutrophils, platelets, and lymphocytes, while PIV also includes platelets, which can be counted among the cells of the innate immune system. The predictive value for disease activity of SII and PIV had also been investigated [20–22]. Additionally, both markers have been investigated for their ability to predict the prognosis of malignancies [23–25]. In the study conducted by Yoshikawa et al. [26], SII and NLR were analyzed in 574 patients with RA, divided into three groups of patients: those in remission, those with low disease activity, and those with high disease activity. As shown in this study, with increasing disease activity, the SII value highly significantly increased; hence SII may be an inflammatory biomarker for assessing disease activity in RA patients. Similarly, in another study conducted in 2021, the SII was examined as an inflammatory marker in RA patients. The study included 109 RA patients and 31 healthy controls. The RA patients were divided into 2 groups: those in remission (DAS28 < 2.6) and those with active disease (DAS28 > 2.6). It was found that SII values were significantly higher in RA patients compared to healthy controls and were associated with disease activity. Consistent with our study, SII values were higher in patients with active RA than in those in remission [13]. The SII and PIV are not only linked to disease activity but also serve as tools for assessing treatment efficacy. A previous investigation revealed that CRP, ESR, and SII effectively tracked treatment responses in patients with RA, with SII demonstrating the most significant predictive capability when compared to CRP and ESR [27]. In our study, which examined the relationship between SII, PIV, and SIRI across active RA, remission, and healthy controls, we found that as SII values increased, the risk of active RA was higher compared to healthy controls. Similar results were found by Liu et al. [12], although their study only examined SII values and did not evaluate other inflammatory parameters or disease activity. Within cancer research, Fucà et al. [28] showed that the PIV score was more effective than various other immuno-inflammatory markers, including the neutrophil-to-lymphocyte ratio, monocyte count, platelet count, and SII, in a logistic regression analysis involving patients with colorectal cancer. A meta-analysis also showed that PIV could serve as a prognostic marker in cancer patients [29]. Our review of the literature revealed just one study that assessed the connection between PIV and RA. Consistent with our results, this research also indicated that PIV was successful in differentiating active RA from remission and control groups [22]. Numerous studies have demonstrated that inflammatory blood markers such as PIV, SII, and SIRI rise during periods of heightened disease activity in RA. The correlation between PIV and SII was explored previously in patients with colorectal cancer, revealing that both markers were significantly higher than those in healthy individuals [30]. In a study on sarcoidosis, PIV and SII values were found to be similar between sarcoidosis patients and controls [31]. In patients with malignant melanoma receiving immunotherapy, PIV and SII were elevated compared to healthy individuals. Additionally, PIV demonstrated notable effectiveness in differentiating between metastatic cases and those classified as stage 1–2 malignant melanoma [25]. In another study on patients with idiopathic membranous nephropathy, PIV and SII were found to be successful in distinguishing between low- and moderate-risk nephropathy [32]. Systemic inflammation response index, recognized as a new biomarker, relies on the proportions of neutrophils, monocytes, and lymphocytes. Prior research has shown that SIRI plays a significant role in the onset and progression of different types of cancerous tumors [15, 16]. Recently, Erre et al. [17] suggested that SIRI may reflect the burden of chronic inflammation in rheumatic diseases rather than being specific to RA. To the best of our knowledge, no additional research exists regarding the clinical use of SIRI in RA. Only one study has assessed SIRI in this context. As previously noted, SIRI indicates the intricate interplay and possible synergy among neutrophils, monocytes, and lymphocytes, which may provide a more objective insight into the relationship between inflammatory and immune responses than NLR and PLR. That study revealed that SIRI was elevated in patients with RA and proposed its potential as a diagnostic biomarker [33]. Our study produced similar results.
Study strenght and limitations
Our research has several strengths. Firstly, it is the first investigation into the relationship among PIV, SII, and SIRI. We assessed active RA patients, those in remission, and healthy controls, analyzing the sensitivity and specificity of the inflammation markers within each group while also comparing their effectiveness against one another. Nonetheless, our study has limitations as it was conducted at a single center with a relatively small participant pool. The treatments administered to the patients were not taken into account, treatment responses were not measured, and there was no follow-up period involved. Future studies with larger populations that take into consideration medication usage are necessary.
Conclusions
This research is the first assessment of the diagnostic and predictive abilities of PIV, SII, and SIRI in RA. The findings revealed that PIV, SII, and SIRI are elevated in individuals with RA and could act as supplementary diagnostic markers. As measures of disease activity in RA, PIV, SII, and SIRI may assist in monitoring treatment effectiveness and enhancing the prognosis for patients.